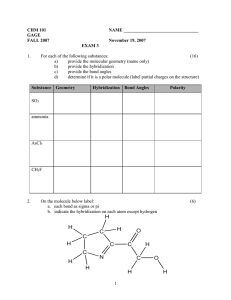
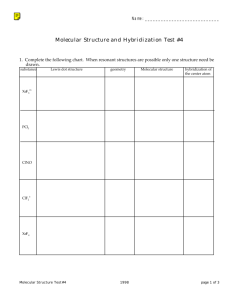
Worksheet 1018 Name _____________________ 18 Jan 2024 For the following molecules provide a Lewis Dot structure for each of the following. For each molecule give both the electronic and molecular geometry. Provide the hybridization of the given atom in the molecule. Is the molecule polar or nonpolar? If polar reflect this in your Lewis Dot structure. 1. O2 Electronic ____________________ Molecular ___________________ O Hybridization _______ Dipole ___________________ 2. BeF2 Electronic ____________________ Molecular ___________________ Be Hybridization _______ Dipole ___________________ 3. CO3−2 Electronic ____________________ Molecular ___________________ C Hybridization _______ 4. NO3− Electronic ____________________ Molecular ___________________ N Hybridization _______ 5. PF6− Electronic ____________________ Molecular ___________________ P Hybridization _______ 1 6. CFCl3 Electronic ____________________ Molecular ___________________ C Hybridization _______ Dipole ___________________ 7. ClF3 Electronic ____________________ Molecular ___________________ Cl Hybridization _______ Dipole ___________________ 8. SO2 Electronic ____________________ Molecular ___________________ S Hybridization _______ Dipole ___________________ 9. SF6 Electronic ____________________ Molecular ___________________ S Hybridization _______ Dipole ___________________ 10. C2H4 (ethene) Electronic ____________________ Molecular ___________________ C Hybridization _______ Dipole ___________________ 2