Fatty Acid
advertisement

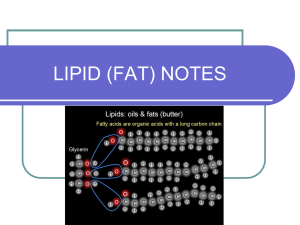
Fatty Acid • A fatty acid is nothing more than a long C-H chain with a carboxyl group (COOH) on the end. • The COOH gives it an acid property. • The 3….dots represent the chain is very long. Glycerol • Glycerol Always looks the same • 3 C’s with 3 OH’s and everything else H’s. To build one lipid molecule, we combine 3 Fatty acids with 1 glycerol by the process of ? What’s What? • Identify the glycerol molecule • The fatty acids Fats make up most of the cell membrane • Remember fats have a hydrophilic head and a hydrophobic tail. Fats are used as an insulator • Fats help keep animals warm Energy • Fats have a lot of energy stored up in their bonds. • That’s why the human body stores fats as an energy source. • When your body needs extra fuel, your body breaks down the fat and uses it as energy. • Sugar give you only a little bit of energy • Fat molecules give off much more energy. Who’s got the power? • Sugar • Fatt Saturated and Unsaturated fats • There are two kinds of fats saturated and unsaturated • Unsaturated fats have at least one double bond (C=C) in one of the fatty acids • Saturated fats have no double bonds. (C-C) Steroids • Steroids are lipids • Since they are lipids they can cross the cell membrane • Are hormones • Have a 4 ring structure Steroid Abuse • Many body builders and athletes use steroids. • They are destroying their internal organs • Cause liver and kidney damage Waxes • Waxes are used to coat and protect things in nature. • Your ears make wax • Plant leaves make waxes to stop evaporation. Element Present Used by organisms Related terms& Info Carbon © Hydrogen (H) Oxygen (O) Only! There is no specific H:O ratio Stored Energy Structure (important part of cell membranes) Saturated fat = C-C bonds are all single Building blocks Fatty acid: of lipids Glycerol: Unsaturated fat = Contain at least one double bond C=C. Is this dehydration synthesis or Hydrolysis? • Lipid + water 3 fatty acids + glycerol Don’t forget to finish your lab! The Mad Scientist