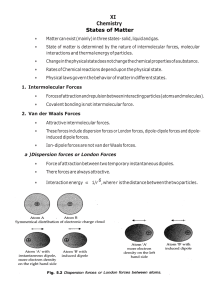
n a P V nb nRT
advertisement

van der Waals Equation for Real Gases 2 P n 2a V nb nRT V where: P is the pressure of the gas V is the total volume of the container containing the gas a is a measure of the attraction between the particles b is the volume excluded by a mole of particles n is the number of moles R is the gas constant, The Ideal Gas Law, PV = nRT, assumes that the molecules that make up the gas have negligible sizes, that their collision with themselves and the wall are perfectly elastic, and that the molecules have no interactions with each other. a and b are constants particular to a given gas (provided in reference tables.) Substance Air Carbon Dioxide (CO2) Nitrogen (N2) Hydrogen (H2) Water (H2O) Ammonia (NH3) Helium (He) Freon (CCl2F2) a (J. m3/mole2) 0.1358 0.3643 0.1361 0.0247 0.5507 0.4233 0.00341 1.078 b (m3/mole) 3.64x10-5 4.27x10-5 3.85x10-5 2.65x10-5 3.04x10-5 3.73x10-5 2.34x10-5 9.98x10-5 The parameter b is related to the size of each molecule. The volume that the molecules have to move around in is not just the volume of the container V, but is reduced to ( V - nb ). The parameter a is related to intermolecular attractive force between the molecules, and n/V is the density of molecules. The net effect of the intermolecular attractive force is to reduce the pressure for a given volume and temperature. When the density of the gas is low (i.e., when n/V is small and nb is small compared to V) the van der Waals equation reduces to that of the ideal gas law. Conditions when real gases are most ideal Conditions when real gases are least ideal Low Pressure High Temperature Small value of n Large Volume High Pressure Low Temperature Large value of n Small volume Small atomic gases with weak IMF’s Large molecular gases with strong IMF’s