THE GASEOUS STATE
advertisement

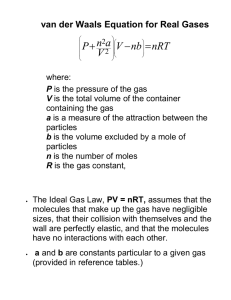
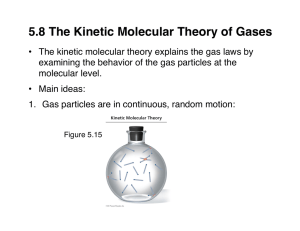
States of Matter Gas: Properties, Laws, KMT Liquid: Intermolecular Forces, VP, Phase Diagrams Solid: Crystal Structure, Types of Solids A Comparison • Gas: low density (d), compressible, takes shape and volume of container. Weak intermolecular forces (IMF). • Liquid: high d, incompressible, takes shape of container but has its own volume. Strong IMF. • Solid: higher d, very incompressible (rigid), takes its own shape and volume. Stronger IMF. Figure 10.1 The Schematic Representations of the Three States of Matter Intermolecular Forces • We will focus on attractive forces between molecules. • These forces are much weaker than chemical bonds that hold atoms together. • And they are nearly nonexistent between gas molecules at room temp. and pressure. • However, they are important in liquids and solids. Properties of a Gas • • • • State of Matter Compressible since molecules are far apart. Takes the shape and volume of container. Forms homogeneous mixtures with other gases. • Pressure is a gas property which tells us about the amount of gas present. Gas Laws • These are empirical laws (based on expts rather than derived from theory) that define mathematical relationships between any two gas properties (P, V, T, n). • For example: If T and n are held constant, what happens to V if you increase P? • V will decreases: Boyle’s Law relates V vs P: V α 1/P or PV = k at constant n and T. Figure 5.15 Increased Pressure due to Decreased Volume Gas Laws (2) • If P and n are held constant, what happens to V if you increase T? • V will increase: Charles’ Law relates V vs T (K): V α T or V/T = b at constant n and P • If P and T are held constant, what happens to V if n increases? • V will increase: Avogadro’s Law relates V vs n: V α n or V/n = a at constant P and T. Figure 5.17 The Effects of Increasing the Temperature of a Sample of Gas at Constant Pressure Why Does an Egg Crack upon Boiling? (p 5) • Charles’ Law: As T increases, V increases. • So what defines the volume that increases with T? V = air sac between porous shell and membrane around egg contents. • As egg ages, the air sac increases. If T increases too quickly, the gas trapped in the sac increases rapidly and cracks the shell. Figure 5.18 Increased Volume due to Increased Moles of Gas at Constant Temperature and Pressure Ideal Gas Law PV = nRT • Combine Boyle, Charles and Avogadro’s Laws • Equation of state for ideal gas; hypothetical state • Note universality of equation; I.e. identity of the gas is not needed. • Limiting law (in the limit of high T and low P~1 atm); this means that as T increases and P decreases, real gases start to behave ideally. Kinetic Molecular Theory of Gases (1) • Gas molecules are far apart form each other and their volumes are • They move constantly, rapidly and randomly in all directions and at various speeds. • There are no intermolecular forces between gas molecules except when they collide. Collisions are elastic. Figure 5.19 Collisions with Walls and other Particles Cause Changes in Movement Figure 5.20 A Plot of the Relative Number of O2 Molecules that Have a Given Velocity at STP Kinetic Molecular Theory of Gases (2) • Measured pressure of gas is due to collisions with walls. • Collisions are elastic. • The average kinetic energy of the gas is proportional to T (K). • KMT explains measurable properties like P, T, V, v and the empirical gas laws. Kinetic Molecular Theory of Gases (3) • Average kinetic energy = [(3/2) RT] α T – KE depends on T only – i.e. KE does not depend on identity of gas (M) • Root mean square velocity – urms = √(3RT/M) where R = 8.314 J/(K-mol) – As T increases, urms [dec, stays the same, inc] – As M increases, urms [dec, stays the same, inc] Figure 5.21 A Plot of the Relative Number of N2 Molecules that Have a Given Velocity at 3 Temperatures