CHEM 1405 Chapter 10 Gases.doc
advertisement

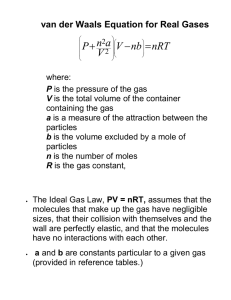
Chem. 1405 Chapter 10 Gases Properties of Gases Gases are compressible They possess low density They do not have a definite shape but assume the shape and volume of their container Their intermolecular distance is large and has negligible intermolecular forces of attraction. Pressure of Gas The pressure exerted by the gas is due to the collision of its molecules on the walls of the container. Pressure = Force / Area (P = F /A) The SI unit of pressure is Pascal (Pa) 1 Pa = 1N/m2 Atmospheric pressure This is the pressure exerted by the gas molecules of the air in the atmosphere 1 atm = 760 mm of Hg = 760 torr =101325 Pa = 14.7psi Atmospheric pressure is measured using the instrument ‘Barometer’ A ‘Manometer’ is used to measure the pressure of gases other than atmosphere. The Gas Laws 1. Boyle’s Law (Relationship between Pressure and Volume of gas) This law states that the pressure (P) of a given mass of gas multiplied by its volume (V) is a constant at a constant temperature. PV = constant or P1V1 = P2V2 (at a constant temperature) 2. Charles’s Law (Relationship between Temperature and Volume) This law states that the volume (V) of a given mass of gas is directly proportional to its temperature (T) in Kelvin scale, at a constant Pressure (P). (As the Temperature increases Volume of the gas increases at constant P) V = (constant) x T V = Constant or V1 = V2 (at constant P) T T1 T2 3. Gay Lussac’s Law (Relationship between Pressure and Temperature) This law states that the pressure (P) of a gas is directly proportional to its temperature (T) in Kelvin scale at a constant volume (V). (As the Temperature increases, the pressure of the gas increases at constant V) P = (constant) x T P = T Constant or P1 = P2 T1 T2 (at constant V) 4. Avogadro’s Law (Relationship between Volume and number of moles or molecules) This law states that equal volumes (V) of all gases at the similar conditions of temperature (T) and pressure (P) contain equal number of molecules (N). VN or V n (Where ‘n’ is the number of moles of the gas) V = (constant) x n V1 = V2 = constant n1 n2 The combined gas Law By combining Boyle’s Law and Gay Lussac’s Law, we get PV = constant T P1V1 = P2V2 This is the combined gas law. T1 T2 The Ideal Gas Equation By combining the Boyle’s law, Charles law and Avogadro’s law, we get the Ideal Gas Equation (PV) /(nT) = constant PV = nRT or n = PV/RT (R = universal gas constant) Value of R R = PV/nT R = (1 atm) x (22.4 L) (1 mol) x (273 K) = 0.0821 L atm mol-1 K-1 (At STP, T = 273K and P = 760mm of Hg or 1 atm. The volume of 1 mole of a gas at STP is 22.4 L) Gas Laws and Molecular mass (M) g PV n= = M RT g RT M= d RT M= V P P Dalton’s Law of Partial Pressures Total pressure of a mixture of gases is equal to the sum of the individual pressures of all the gases in the mixture. The individual pressures are called partial pressure. Ptotal = PA + PB Partial pressure of Gas A (PA ) = nART / V Partial pressure of Gas B (PB ) = nBRT / V If the total pressure of the mixture were given, then the partial pressure of gas A & B would be, PA = Pmix x mole fraction of A PB = Pmix x mole fraction of B Mole fraction of a component in the mixture = Number of moles of that component Total Number of moles Kinetic molecular Theory of Gases Assumptions 1. A gas is composed of very minute particles called molecules. 2. The molecules are in a state of constant motion in random directions. During their movement they collide with each other and also with the walls of the container. 3. The molecules are perfectly elastic and the collisions do not result in any loss of energy. 4. There are no intermolecular forces of attraction between molecules. 5. The volume occupied by a molecule is negligible when compared with the total volume of the gas. 6. The average kinetic energy of the molecules is directly proportional to the temperature of the gas in Kelvin. According to Kinetic theory of gases, PV = 1 / 3 mNu2