Ideal Gas Equation Lesson Plan: Chemistry
advertisement

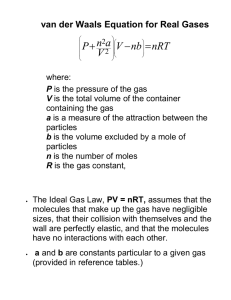
Lesson Plan Lesson: Ideal Gas Equation Aim: To investigate the behaviour of an ideal gas. Learning Outcomes : At the end of the lesson, students will be able to : 1. used the ideal gas equation : PV = nRT in calculations. 2. state and explain the basic assumptions of the kinetic theory of gases as applied to an ideal gas. 3. explain qualitatively how gases approach ideal behaviour at low pressures and high temperatures. 4. explain the limitations of ideality at very high pressures and very low temperatures. Assumed prior knowledge : Students should already : 1. be familiar with Boyle’s Law and Charles’ Law. Underlying Principles 1. 2. Making the invisible, visible. Teaching students how to manage data and interpreting it graphically. Time taken to complete the activities : 80 minutes Differentiation Questions in the student notes are designed to enable all students to complete the activity. The pop-up answers are provided for the students to view when they have considered their responses. Worksheet questions include questions that require recall, understanding and application of the new concepts learned. © 2003 Ministry Of Education Malaysia. All Rights Reserved. 1 Development of Lesson : No. 1 2 Steps Set Induction. (Ascertaining prior knowledge and introducing lesson topic for the day). Strategy • Teacher to get students to recall Boyle’s Law and Charles’ Law. • Teacher to point out these laws can be combined to form the Ideal Gas Equation. Student Activity Teacher to go through Activities 1 & 2 with students. • Activity 1 : Ideal Gas Equation Get students to derive the ideal gas equation from Boyle’s and Charles’ Laws. Students to use the ideal gas equation in calculations including calculations to find relative molecular mass. • Activity 2 : Ideal and real (non-ideal) gases. Get to compare the PV vs P graphs for three non-ideal gases [hydrogen, nitrogen and carbon dioxide] with the ideal gas graph and find out the explanation for these graphs in terms of intermolecular forces and the volume of the molecules. Resources • Courseware 3 Evaluation • Students to answer questions in the student worksheet on their own. • Worksheet 4 Extension activity • Students to go through the extension activities on their own. • Website and References. © 2003 Ministry Of Education Malaysia. All Rights Reserved. 2 Worksheet Answers 1. Ideal Gas Equation 1.1 The ideal gas equation is PV = nRT. 1.2 Boyle’s and Charles’ laws. 1.3 a. n = PV/RT = (2.0 x 104 Pa x 2.0 x 10-2 m3) / [8.31J K-1 mol-1 x (0 + 273) K] = 0.18 mol b. P = nRT/ V = [0.18 mol x 8.31 J K-1 mol-1 x (35 + 273) K] / 2.0 x 10-2 m3 = 2.3 x 104 Pa c. n = PV/RT = (0.5 x 104 Pa x 2.0 x 10-2 m3) / [8.31J K-1 mol-1 x (20 + 273) K] = 0.041 mol The number of mols of nitrogen gas used = 0.18 – 0.041 = 0.14 mol 1.4 V = nRT/P = [1.0 mol x 8.31 J K-1 mol-1 x (130 + 273) K] / (101 x 103) Pa = 0.032 m3 = 3.2 x 104 cm3 1.5 a. PV = nRT = (m/Mr) RT Mr = mRT/PV b. i. Mr = (0.2590 g x 8.31 J K-1 mol-1 x 273 K) / (101 x 103 Pa x 100.0 x 10-6 m3) = 58.2 b. ii. (C2H5) x n = 58.2 29 n = 58.2 n=2 Molecular formula of the gas is C4H10. © 2003 Ministry Of Education Malaysia. All Rights Reserved. 3 2. Ideal and Real (non-ideal) Gases 2.1 The size of the gas molecule is negligible (very small) compared to the size of its container, and there is no interaction (attraction or repulsion) between the molecules. 2.2 Real gases obey the ideal gas equation under certain conditions. a. Hydrogen or oxygen or helium or carbon dioxide, (etc) b. At low pressures and high temperatures. At low pressures, the gas molecules are far apart. Under this condition, its molecular size and intermolecular interaction are insignificant. At high temperatures, the kinetic energies of the gas molecules are high. The fast moving molecules have little attraction for each other. c. At high pressures and low temperatures. At high pressures, the gas molecules are close together. Under this condition, its molecular size and intermolecular interaction are significant. At low temperatures, the kinetic energies of the gas molecules are low. The slow moving molecules interact with each other significantly. 2.3 a. A graph of PV/nRT vs P for (a) ideal gas, (b) a gas that deviate positively at all pressures, and (c) a gas that deviates negatively at low pressure: b. All gases deviate positively at high pressures because the molecules that are too close together repel each other. © 2003 Ministry Of Education Malaysia. All Rights Reserved. 4 2.4 A graph of PV/nRT vs P for methane at (a) 25 oC and (b) 750 oC. c. At 25 oC, methane gas shows negative deviation from the gas law at low pressures and positive deviation at high pressures. At this low temperature, the gas molecules have low kinetic energy. When the pressures are low, the molecules are far apart. Thus, the size of the molecules is insignificant, but there exists intermolecular attraction. At high pressures, the slow moving molecules which are too close together repel each other. At 750 oC, methane gas shows positive deviation from the ideal gas law at all pressures. At this high temperature, the gas molecules have high kinetic energy. The fast moving molecules do not attract each other. Instead, they repel each other at all pressures. © 2003 Ministry Of Education Malaysia. All Rights Reserved. 5