Andrew Rosen 10.1 – Characteristics of Gases and 10.2 - Pressure
advertisement

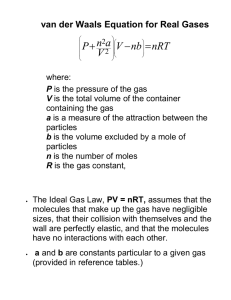
Andrew Rosen 10.1 – Characteristics of Gases and 10.2 - Pressure Manometer – Measures differences in pressure between atmospheric pressure and that of a gas (invented by Toricelli) Closed: Open (atmospheric pressure > gas pressure): Open (gas pressure > atmospheric pressure): 10.3 – Gas Laws *Note: Temperatures are in kelvins* Boyle’s Law: [indirect relationship when T and n are constant] Charles’ Law: [direct relationship when P and n are constant] Avogrado’s Law: Combined Gas Law: [direct relationship when P an T are constant] [when n is constant] 10.4 – The Ideal-Gas Equation Ideal-Gas Equation: PV=nRT [R = 0.08206 ] d= *Note: 1m3 = 1000L… use the conversation page* 1 mol of ideal gas occupies 22.4L at STP 10.5 – Further Applications of the Ideal-Gas Equation and 10.6 – Gas Mixtures and Partial Pressures Air has 78% N2, 21% O2, 1% Ar “ICE Boxes” can be set up with units of volume when pressure and temperature are held constant because there is a direct relationship between moles and volume “ICE Boxes” can be set up with partial pressures when volume and temperature are held constant because there is direct proportionality with partial pressure and moles Dalton’s Law: Mole Fraction: For gas mixtures, mole% = % of partial pressure If a gas mixture is separated into pure components (with constant P), mole% = % of volume Andrew Rosen For gas collection over water, *Be careful: If it gives percentages by “mass” you need to make them mole fractions via moles* 10.7 – Kinetic-Molecular Theory of Gases 1) Volumes are assumed to be zero for particles 2) Particles are in constant, random motion (pressure changes) 3) Particles exert no force on one another (do not repel/attract) 4) Energy can be transferred during collision, but the average KE is the same with constant T (elastic collisions) Absolute Temperature: Average KE of the molecules of a gas Root-Mean-Square (RMS) speed: Speed of molecules possessing average KE √( ) [R = 8.3145 ] *Need R value that has Joules in it* *Molar mass is in kg] = Most probable speed (top of bell-curve), √ = average speed (greater than ), = RMS speed (greater than ) [M is MOLAR MASS in kg!!!] 10.8 – Effusion and Diffusion Effusion – Escape of gas molecules through a tiny hole Diffusion – Spread of one substance throughout a space or second substance Graham’s Law of Effusion: √ Note: Rate is inversely proportional to time [ ] or √ If it says it takes 3.6 times longer, think about it. That’s not rate. That’s time. If it goes 3.6 times the rate of the other one, it’s going faster, not slower! Lighter molecules diffuse faster and diffuse faster with increasing temperature Mean Free Path: Average distance traveled by a gas molecule between collisions [increases with decreasing pressure] Convection: Mixing of gases due to an imbalance of temperatures [diffusion is slower than convection] 10.9 – Real Gases: Deviations from Ideal Behavior Real molecules have finite volumes and attract one another [still no repulsive forces in gas phase] Deviations from ideal behavior occur at low temperatures and high pressures Andrew Rosen Happens because slower molecules stick together better (low temp) and have closer contact (high pressure) Liquefaction of gases is easier to achieve at high pressures Van der Waals Equation: ( ) [don’t need to memorize but need relationships] Coefficient a accounts for molecular attractions (van der Waals forces) and increases with polarity and molar mass Coefficient b accounts for finite size of gas molecules (van der Waal radius) and increases with size of molecule Higher a and b values increase deviation from ideal behavior [leading to easier liquefaction]