Dehydration of Alcohols to form Ethers
advertisement

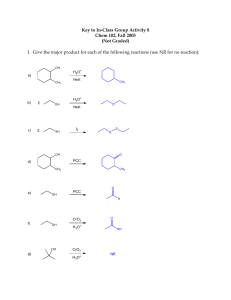
Dehydration of Alcohols to form Ethers • Simple, symmetrical ethers can be formed from the intermolecular acid-catalyzed dehydration of 1° (or methyl) alcohols (a “substitution reaction”) • 2° and 3° alcohols can’t be used because they eliminate (intramolecular dehydration) to form alkenes OH H3O+ + OH + O heat H2O •Unsymmetrical ethers can’t be made this way because a mixture of products results: + OH + CH3-OH H3O heat + O + O O Mechanism of Formation of Ethers from Alcohols • First, an alcohol is protonated by H3O+ • Next, H2O is displaced by another alcohol (substitution) • Finally, a proton is removed by H2O to form the product H OH H O + + O H H O H H H H OH O O + O + H H H H H O H + H H O O + O H H Oxidation of Alcohols • Recall that oxidation is a loss of electrons and reduction is a gain of electrons • However, red-ox does not always involve ions • Oxidation can also be defined as a gain in bonds to oxygen • This is because O is more electronegative than all other elements (besides F), so it removes electron density from any element with which it forms a covalent bond • Primary alcohols can be oxidized to aldehydes and carboxylic acids and secondary alcohols can be oxidized to ketones [O] O [O] O OH H OH [O] O OH Oxidation of Primary and Secondary Alcohols • Primary alcohols are initially oxidized to aldehydes, but aldehydes are easily oxidized to carboxylic acids • In order to stop the reaction at the aldehyde a very mild reducing agent must be used • The most common reagent is PCC (pyridinium chlorochromate) • Other oxidizing agents (like CrO3/H3O+) will oxidize primary alcohols all the way to carboxylic acids • Secondary alcohols can be oxidized with either reagent PCC O OH OH H CrO3 OH H3O+ PCC or CrO3/ H3O + O OH O Oxidation of Thiols • Oxidation can also be defined as a loss of bonds to hydrogen • This is because H is less electronegative than all other nonmetals (besides P which is the same), so adds electron density to any element with which it forms a covalent bond • Thiols can be oxidized to disulfides using I2 (or Br2) • In proteins, disulfide bonds between sulfur-containing amino acids (cysteines) are a major factor in preserving their shape SH + I2 SH S S + 2HI