Study Guide-Lect Exam 1- Part 2.doc
advertisement

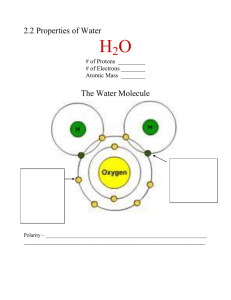
Exam 1 Study Guide Part 2 of 3 Parts Biol1406._______(FA’15) Isotopes and how they result Define isomers The types of bonds within and between water molecules Explain water polarity and what is it due to The difference between single, double, & triple covalent bonds The cause of hydrogen bond formation Define the following atomic and subatomic particles based on their different characteristics: o An atom o An anion o A cation o A molecule o A neutron A characteristic of water that is not found in oils The two main types of chemical reactions and other terms identifying them The specific base-pairing of nucleic acids The building blocks of nucleic acids Identification of commonly known sugars based on their structural units/monomers Water properties associated with hydrogen bonding The experimental proof of water surface tension The three physical changes accompanying freezing of water into ice The relationship between acidity, hydrogen ion concentration, and the pH Endorgenic vs exorgenic reaction Define an alkaline solution in terms of pH & hydrogen ion concentration Distribution of electrons on energy shells & determination of reactivity of the element The name of long chains of macromolecules (polymers vs monomers) The function of enzymes The number and distribution of subatomic particles The different components of a nucleotide The valence shell & its relationship to chemical reactivity The four types of bonds, their relative strength, and the way they form Identify the different classes/names of macromolecules The macromolecule(s) that function: o as an immediate source of energy o as a stored form of energy o as carrier of protein synthesis information o in growth and tissue repair