Chapter 22 Compounds Between Metals and – the Ionic Bond Non-Metals
advertisement

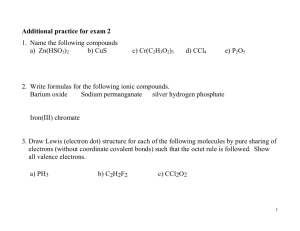
Chapter 22 Compounds Between Metals and Non-Metals – the Ionic Bond Called Salts Band Gap semiconductors Near the boundary between metals and non-metals Conduction and valence bands do not overlap conduction band band gap valence band E= h times frequency; c= wavelength times frequency Red longer wavelength means lower frequency, lower energy. Red photons are emitted from a warm LED; orange photons are emitted when the LED is very cold. What happened? A. The band gap shrinks when the semiconductor gets cold. B. No “red” electrons get kicked upstairs to fall back down. C. The band gap increases when the semiconductor gets cold. Did you read chapter 22 before coming to class? Or do you have a Halloween costume? Yes b) No a) Which element will react with water in a way most similar to Na? O b) Cl c) Li d) Hg a) Which of the following has the highest ionization energy a) b) c) d) Na (Z=11) Al (Z=13) Cl (Z=17) Ne (Z=10) Ionization Energies Nobel gasses have largest ionization energies. Alkali metals have the least. Compare and Contrast: Ionic Compounds vs Metals Network Solids High melting T’s Brittle solids Don’t conduct heat and electricity in solid Often colorless and usually transparent in big chunks (White when powdered) WHY? Network Solids High melting T’s Malleable Good conductors of heat and electricity in solid Opaque Why??? Many closely spaced energy levels with mobile electrons Compare and Contrast: Metals vs Non-Metals Metals Large atoms Few valence electrons Low ionization energies Non-metals Small atoms Many valence electrons High ionization energies Why do metals and non-metals react? Principles of reactivity: materials react to lower energy and increase entropy of universe How can energy be lowered? Metals lose valence electrons Non-metals gain valence electrons Energy levels not drawn to scale. Cl levels much lower in energy! Transfer of electron from Na to Cl is downhill energetically What about entropy change? 2Na + Cl2 = 2NaCl + lots of heat and light Na – speck Cl2 yellow gas sand at bottom Quicktime Video of reaction Heat and light – cause an increase in entropy of the surroundings What are the products? IONS neutral atom Positive ion Neutral Cl atom Negative Cl ion Negatively charged Positively charged Chloride ions Sodium ions 35 protons, 36 electrons (11 protons, 10 electrons) Electrons belong to individual ions; they are not shared among ions. Examples of Ionic Compounds NaCl Al2O3 Na2O Ions: same charges Ions: different Ions: similar sizes, and similar sizes charges and sizes but different charges Describe the structure of each compound: Do ions of one type cluster together? What type of ion immediately surrounds a given ion? How do the answers to these two questions relate to the electric force law? What prediction could you make about the arrangement of ions in any ionic compound? Which structure is the form adopted by Al2O3 in nature? A. B. What factor most likely prohibits this structure? Strong repulsive forces between negative O ions and between positive Al ions B. Not electrically neutral C. Low entropy organization A. Compare & Contrast Energy Levels IONIC COMPOUND ENERGY LEVELS few levels -- spaced very far apart Metal Energy Levels many closely spaced levels spread out over many nuclei Energy Levels Explain Ionic Salts Transparent in Visible Region of Spectrum – But absorb in UV Metals Opaque – absorb in IR, Visible and UV (exception: salts containing certain transition metal ions absorb in visible region.) l20newcrystal.swf l20photon.swf Compare and Contrast Electron Locations & Mobilities IONIC COMPOUNDS + + + + + + + + + Electrons – localized on individual nucleus (spherically shaped electron clouds in most ions) METALS AND ALLOYS Sea of Electrons – mobile; electron density is spread out over many nuclei, delocalized. How does the model explain properties of salts (ionic compounds) ? High melting and boiling temperatures? Strong attractions between + and – ions Attractive forces act over fairly large atomic distances Brittleness? Strong repulsions when ions with like charge come together; material shatters to relieve the stress. ELECTRICAL CONDUCTIVITY: Flow of electricity requires charge carriers that are free to move. In solid, ions are fixed rigidly in place. No Current can flow! Melting frees up ions so that they can move, completing the electrical circuit. Dissolving salt in water also frees up ions. Current flows! Even More Properties Why are some ionic materials colored? Because they contain “transition” metals with more energy levels for electrons • Example: Ruby Absorbs blue photons Absorbs green photons Doesn’t absorb red photons: reflects red What Ions Usually Form? Using the Periodic Table to make predictions. Valence Electrons of Main Group Elements Unreactive noble gases don’t form ions. Metals LOSE their valence electrons. Non-metals GAIN enough valence electrons to become “noble”. The octet rule Atoms will most likely form an ion that has the ns2np6 configuration of the closest noble gas atom. Metals take on this configuration by losing electrons Non-metals take on this configuration by gaining electrons Families Chlorine and Fluorine will form the same types of compounds since their valence electrons are the same number and same orbital type. 3d 3p 3s 3d 3p 3s 2p 2s 2p 2s 9F 17Cl 1s 1s When Mg loses its two electrons, it has the same valence electron configuration as A. He, 1s2 2 6 B. Ne, 2s 2p 2 6 C.Ar, 3s 3p D.Kr, 4s24p6 When Br gains one electron, it has the same valence electron configuration as 1. 2. 3. 4. He, 1s2 Ne, 2s22p6 Ar, 3s23p6 Kr, 4s24p6 Beryllium (Be) will most likely form an ion with what charge? a) b) c) d) -1 -2 +1 +2 What would the chemical formula for magnesium fluoride (a salt of Mg and F) be? a) b) c) d) MgF Mg2F MgF2 MgF3 Ionic compounds are neutral (no net charge). What are the ionic charges in the following compounds? NaCl Na+1 and Cl-1 KBr K+1 MgF2 Mg+2 Al2O3 Al+3 and Br-1 and F-1 and O-2 Naming convention for salts The metal comes first with its name unchanged The nonmetal comes second, with the suffix “ide” appended