Molecular Models & Covalent Bonding Worksheet
advertisement

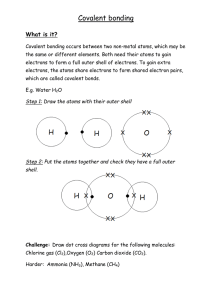
Molecular Models Atoms form bonds to become molecules and compounds because of their electrons. Today we will be modelling covalent bonds which occur when electrons are shared between atoms. Complete the following table. Element Oxygen Electron configuration (written and diagram) Number of extra electrons the atoms needs to gain (ionic bonding) or share (covalent bonding) 2.6 Carbon Hydrogen Nitrogen Examine the atoms that represent oxygen, carbon, hydrogen and nitrogen. What do the holes in the balls represent? Atoms stay in molecules because once they are in these, they have full outer electron shells, and are thus stable. This means that for a stable compound to be formed, the entire valence (outer) electron shell must have been filled (no holes left unfilled in the molecular models). Make and draw the following compounds, and express their structural formula. Chlorine Cl2 Oxygen O2 Nitrogen N2 Carbon dioxide CO2 Methanal CH2O Ethanol CH3COOH (Hints: one of the oxygen atoms is joined only to a single carbon atom).