ddd

Why all atoms are not stable?
Only those atoms having 8 electrons in the last shell are stable.
Example: Helium, Neon (2,8), Argon (2,8,8)
Atoms need to have eight electrons in the outer shell for stability.
Chemical bonding:
A chemical bond i s formed due to the attraction between two atoms or ions.
Formation of chemical bonds make atoms stable by straining stable electron striation in the outermost shell.
There are 3 types of chemical bonding:
1) Ionic bonding
Made up of ions.
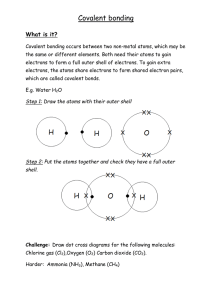
2) Covalent bonding
This kind of bonding takes place when non-metal atoms bond together.
Non-metal atoms share electrons between them.
So electron pairs (each from different atoms, this pair is not used by both atoms) are shared by atoms. One pair rep resents a “covalent bond”, the numbers of pairs are formed depending on who many more atoms are needed.
If one pair, it is a single bond, two pairs double bond and three pairs, triple bond.
3) Metallic bonding
Noble gas has full atom shell, the atom is stable.
Metals - One the left hand side of the periodic table. They all conduct electricity (1-3 electrons)
Non-Metals - On the right hand side of the periodic table. (more than 3 electrons)
Metalloids- Elements present in between metals and non-metals
Ionic bonding:
An ionic bond is formed when a metal atom bonds with a non-metal atom.
Metal atom loses those 1 or 2 or 3 electrons from the outermost shell.
Remember, atoms are neutral.
When electrons (negative charges) are lost, positive charges dominates and the atoms becomes positive ion.
Non-Metal atoms gain electrons to make the the outermost shell full.
When a non-metal atom gains electron (s), the become negative ion.
Once the ions are formed, they attract each other (+ and - attraction). This attraction of positive an negative ions is called ionic bonding.
Na - e = Na +
Cl + e = Cl -
Na + Cl - = NaCl
0
Represent an ion in a square bracket
But electron just draw it normally.
Ca - 2e = Ca2+
2Cl + 2e = 2 Cl -
Ca 2 + 2Cl - = Ca Cl 2
Properties of covalent compounds
Covalent compounds generally have much lower melting and boiling points.
Covalent compounds do not pass electric current when melted.
They are generally liquids or gases at room temperature.
2)