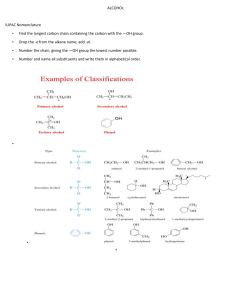
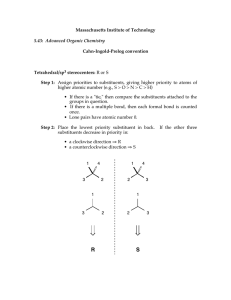
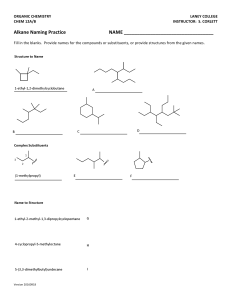
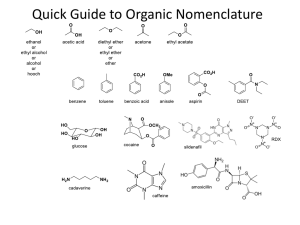
Organic Chemistry: Nomenclature Summary IUPAC SYSTEM OF NAMING COMPOUNDS 1. Select the longest continuous chain of carbon atoms for the basic name. 2. Number the carbons in the chain from the end nearest the first branch (to give lowest set of numbers). 3. Identify substituents. If more than one substituent of the same kind is present, use the prefixes “di”, “tri”, “tetra”. 4. Locate the substituents by the number of the carbon to which they are attached. 5. Put substituents in alphabetical order (multiplier prefixes do not count). 6. Separate numbers from letters by a dash and write the whole name as one word with the basic name at the end. IUPAC SYSTEM OF NAMING ALKENES 1. Select the longest continuous chain of carbon atoms which contains both carbons of the double bond for the basic name. 2. Change the ending to –ene from the –ane of the corresponding alkane 3. Number the carbon atoms in the chain from the end nearest the first carbon of the double bond. 4. Locate the position of the double bond by the number of the first carbon involved in the double bond. 5. Name substituents as with alkanes 6. In cyclic system number the carbons of the double bond as 1 and 2. IUPAC SYSTEM OF NAMING CARBOXYLIC ACIDS Select the longest continuous chain of carbon atoms which contains the carbon bearing the carboxylic group. 2. Change the ending to –oic from the –ane of the corresponding alkane. 3. The carboxylic carbon atom is assigned number 1. 4. Locate and name substituents as with alkanes. SUBSTITUENTS 1. 2. 3. 4. 5. F- fluoro Cl- chloro Br- bromo I- iodo IUPAC SYSTEM OF NAMING ALKYNES Select the longest continuous chain of carbon atoms which contains both carbons of the triple bond for the basic name. Change the ending to –yne from the –ane of the corresponding alkane. Number the carbons in the chain from the end nearest the first carbon of the triple bond. Locate the position of the triple bond by the number of the first carbon involved in the triple bond. Name substituents as with alkanes. CARBOXYLIC ACIDS (cont’d) 1. 1. 2. 3. 4. 5. 6. IUPAC SYSTEM OF NAMING ALCOHOLS Select the longest continuous chain of carbon atoms which contains the carbon bearing the hydroxyl group. Change the ending to –ol from the –ane of the corresponding alkane. Number the carbons in the chain from the end nearest the carbon bearing the –OH. Locate the position of the –OH by the number of the carbon to which it is attached. Name substituents as with alkanes. Hydroxyl (-OH) groups have priority over double bonds in terms of numbering. ESTERS Nomenclature: alkyl of alcohol part + alkyl of carboxylic acid part + oate carboxylic acid alcohol