Chm 123 Name: Evaluation Quiz
advertisement
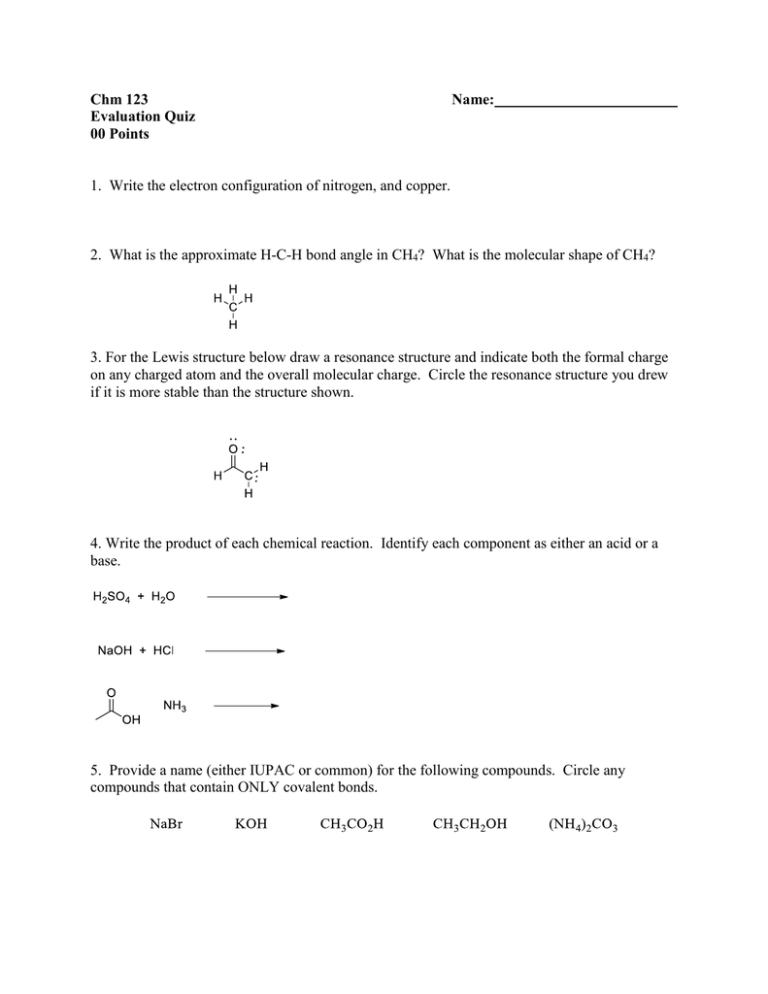
Chm 123 Evaluation Quiz 00 Points Name: 1. Write the electron configuration of nitrogen, and copper. 2. What is the approximate H-C-H bond angle in CH4? What is the molecular shape of CH4? 3. For the Lewis structure below draw a resonance structure and indicate both the formal charge on any charged atom and the overall molecular charge. Circle the resonance structure you drew if it is more stable than the structure shown. 4. Write the product of each chemical reaction. Identify each component as either an acid or a base. 5. Provide a name (either IUPAC or common) for the following compounds. Circle any compounds that contain ONLY covalent bonds.









