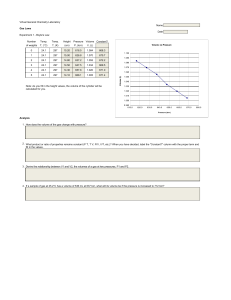
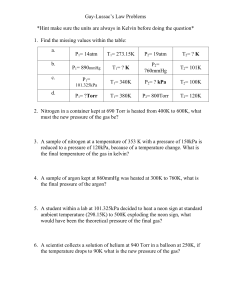
Quiz Example
advertisement

Quiz-A Ch. 10 Gases Name:______________ Honor Pledge: You MUST show all of your work or no credit will be given for the problem. 1. 2. Convert the following pressures: a. 780 Torr = ________ mmHg c. 700 Torr = _________kPa b. 698 mmHg = ________Pa d. 100kPa = __________atm Calculate the new volume when 450.0mL of a gas is cooled from 60.0°C to 20.0°C at constant pressure. Name the gas law ________________ 3. A volume of gas is originally at a temperature of 20°C and has a pressure of 0.930atm. If the volume remains constant and the temperature increases to 30°C, calculate the new pressure. Name the gas law_______________ 4. If a 2.0 L sample of gas experiences a decrease in pressure from 1.74atm to 0.555atm at 25°C, what is the resulting volume (in L) at 25°C? Name the gas law________________ 5. If at constant temperature, the volume of a flask equipped with a moveable piston is reduced to ¼ its original volume, what must happen to the pressure?