Titration Lab Report - Walker Singleton
advertisement
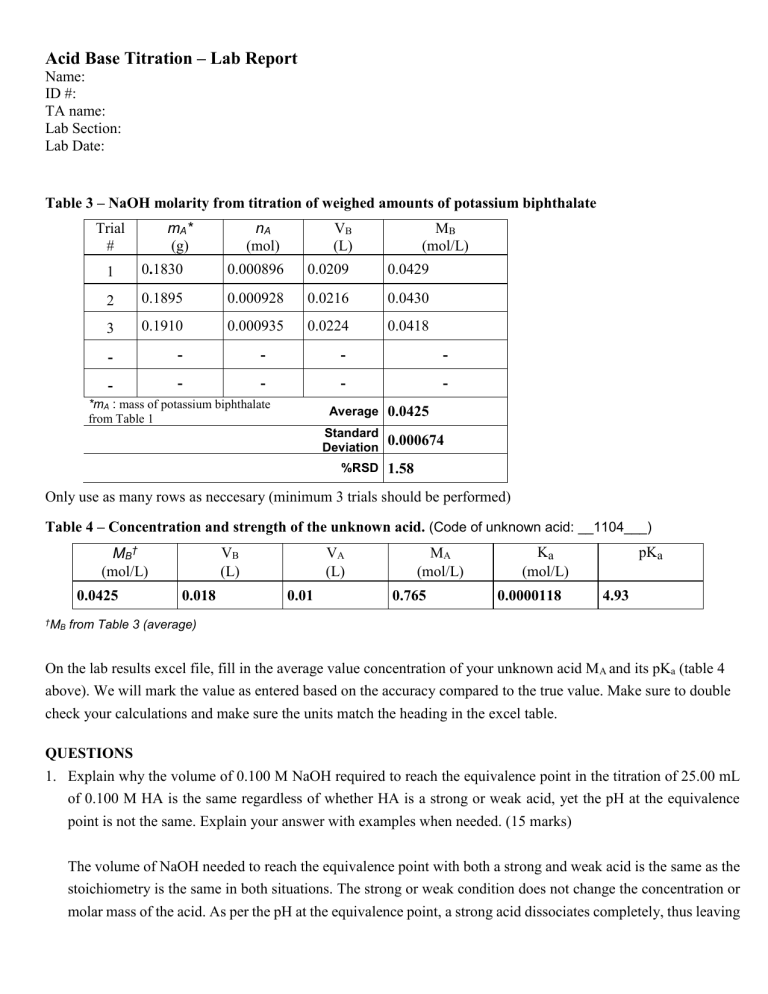
Acid Base Titration – Lab Report Name: ID #: TA name: Lab Section: Lab Date: Table 3 – NaOH molarity from titration of weighed amounts of potassium biphthalate mA* (g) Trial # nA (mol) VB (L) MB (mol/L) 1 0.1830 0.000896 0.0209 0.0429 2 0.1895 0.000928 0.0216 0.0430 3 0.1910 0.000935 0.0224 0.0418 - - - - - - - - - - *mA : mass of potassium biphthalate from Table 1 Average Standard Deviation %RSD 0.0425 0.000674 1.58 Only use as many rows as neccesary (minimum 3 trials should be performed) Table 4 – Concentration and strength of the unknown acid. (Code of unknown acid: __1104___) MB† (mol/L) 0.0425 †M B VB (L) 0.018 VA (L) 0.01 MA (mol/L) 0.765 Ka (mol/L) 0.0000118 pKa 4.93 from Table 3 (average) On the lab results excel file, fill in the average value concentration of your unknown acid MA and its pKa (table 4 above). We will mark the value as entered based on the accuracy compared to the true value. Make sure to double check your calculations and make sure the units match the heading in the excel table. QUESTIONS 1. Explain why the volume of 0.100 M NaOH required to reach the equivalence point in the titration of 25.00 mL of 0.100 M HA is the same regardless of whether HA is a strong or weak acid, yet the pH at the equivalence point is not the same. Explain your answer with examples when needed. (15 marks) The volume of NaOH needed to reach the equivalence point with both a strong and weak acid is the same as the stoichiometry is the same in both situations. The strong or weak condition does not change the concentration or molar mass of the acid. As per the pH at the equivalence point, a strong acid dissociates completely, thus leaving a neutral pH of 7. A weak acid does not completely dissociate and continues to ionize into OH-, raising the pH into basic areas. 2. Show all the steps and calculations involved to complete the first row of Table 3 and 4. What is the identify of your unknown acid? (20 marks) 1 𝑚𝑜𝑙 nA = 204.22 𝑔= 0.000896 moles MB = Ka = 𝑛𝐴 𝑉 0.000896 = 0.0209 [𝐻30+][𝐴−] [𝐻𝐴] = 0.0429 M = 0.000018 mol/L pKa = -log(ka) = 4.93 This pH indicates the presence of acetic acid. 3. At the beginning of a titration to standardize the NaOH solution, Student A adjusted very carefully the initial burette volume to 0.00 mL, but he did not notice an important air bubble in the tip of the burette. At the end of the titration, the air bubble is gone. Explain the effect of that mistake on the calculated molarity MB. (Will the experimental MB calculated by Student A be higher or lower than the true MB value?) (5 marks) The air bubble will lead to a miscalculation of volume added, as the student will think they added more NaOH than they actually did. This will lead to a larger volume, which in turn will decrease in molarity. Thus the calculated molarity will be lower than the actual value. Once you are complete, remember to save as .pdf file and submit to MyCourses. Double check your submission to make sure that you have submitted the correct file. (Sometimes students accidentally submit the empty template, a corrupt file or the wrong file altogether, double check the file that you have submitted to make sure this doesn’t happen to you.) 2










