Acid-Base Titrations Titrations are analytical procedures that can determine an unknown
advertisement

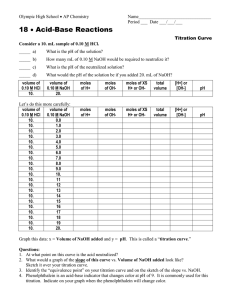
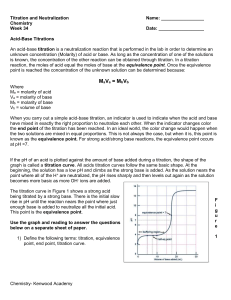
Acid-Base Titrations Titrations are analytical procedures that can determine an unknown concentration. The equivalence point of a titration is reached when the moles of acid equal the moles of base. This idea can be expressed as: na = nb or MaVa = MbVb. The concept is to accurately measure three of the four quantities and solve for the unknown. A titration is performed where a strong base (1 M) is added to 20 mL of a strong acid (1 M). The pH is plotted against volume of base and the following plot is obtained. 14 pH 7 0 0 10 20 Volume of base (mL) 30 40 If a strong base is added to a weak acid the titration curve will alter in several ways. 1) the original pH of the weak acid will be higher than a strong acid 2) the first half of the curve will have a buffering region – this is a region where a large quantity of base will only change the pH a relatively small amount – buffers are created by the presence of an equilibrium established between a weak acid (eg. CH3COOH) and its conjugate base (CH3COO1-). The buffer capacity is exceeded when [CH3COOH] = 0. The curve will then resemble the strong acid/base graph. 3) the pH at the equivalence point will be higher than 7.0