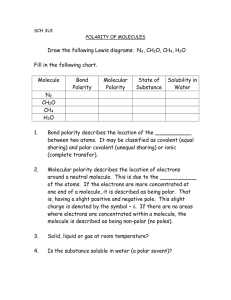
DAILY LESSON PLAN/ DLL Duration: 1 Hour & 30 minutes/Day Total No. No. of Student per of Students class: 34 Modality SCHOOL MNCHS GRADE LEVEL/ SECTION TEACHER CEDIÑO, OLGA VICTORIA B. SUBJECT PHYSICAL SCIENCE QUARTER THIRD QUARTER SEM SECOND TEACHING DATE TIME APRIL 3, 2023 2:00-3:30PM FACE-TO-FACE TEACHING DAY I. OBJECTIVES: A. Content Standards GRADE 12 - COOPERATION MONDAY At the end of the session 85% of the learners will be able to: 1. Demonstrate an understanding of: a.) How the uses of different materials are related to their properties and structures. The learners shall be able to explain: B. Performance Standards C. LEARNING COMPETENCIES OBJECTIVE/S SPECIFIC OBJECTIVE/S 1. how the uses of different materials are related to their properties and structures. 1. Determine if a molecule is polar or non-polar given its structure. (S11/12PS-IIIc-15) 2. Relate the polarity of a molecule to its polarity. (S11/12PS-IIIC-16) Specific Objectives: At the end of the session learners are expected to: a.) b.) c.) d.) e.) f.) Define electronegativity. (cognitive) Find the electronegativity of elements in the periodic table. (cognitive) Explain the VESPR Theory. (cognitive) Draw the geometry of molecules using the VESPR Theory. (psychomotor) Distinguish between polar and non-polar molecules. (cognitive) Relate the properties of molecules to its polarity and provide an overview of the proof for the Big Bang model. (affective) INTEGRATION A. Subject: Grade 9 Science – 2nd Quarter (within) 1. Learning Competencies: Explain the formation of ionic and covalent bonds. LC Code: S9MT-IIa-13 2. Learning Competencies: Recognize different types of compounds (ionic or covalent) based on their properties such as melting point, hardness, polarity, and electrical and thermal conductivity. LC Code: S9MT-IIb-14 3. Learning Competencies: Explain how ions are formed. LC Code: S9MT-IIe-f-16 B. Subject: TVL Home Economics – Bread and Pastry (NCII) and Food Processing (NCII) (across) CLASSROOM OBSERVATION TOOL1 P a g e 1|9 Learning Competencies: Prepare variety of pastry products according to standard mixing procedures/formulation/ recipes and desired product characteristics LC Code: TLE_HEBP9- 12PP-IIa-g-4 Learning Competencies: Monitor accurate of measuring/dispensing equipment to identify variation in operating conditions according to production requirements LC Code: TLE_AFFP9- 12NB -IIIf-j-2 II. PHYSICAL SCIENCE How the properties of matter relate to their chemical structure III. CONTENT LEARNING RESOURCES A. Reference 1. Curriculum Guide Page 2. Textbook/ Reference 3. Other learning resources. K to 12 BASIC EDUCATION CURRICULUM SENIOR HIGH SCHOOL – CORE SUBJECT K to 12 Senior High School Core Curriculum –Physical Science August 2016 Page 3 of 17 Code Book Legend Sample: S11/12PS-IIIa-1 IV. PROCEDURES A. Reviewing previous lesson or presenting the new lesson. Physical Science – Grade 11/12 (ADM Version) Quarter 1 – Module 2: Exploring Polarity of Molecules and Its Properties (1) Whitten, K.W., Davis, R.E., Peck, M.L., & Stanley, G.G., (2005). General Chemistry 7th ed. Singapore: Thomson/Brooks/Cole. (2) (2) Atkins, P. W. Chemical Bonding. (2016). In Encyclopedia Britannica. Retrieved February 20, 2016 from http:// www.britannica.com/science/chemical-bonding/Intermolecularforces (3) Berstein, R., Carpi, A., (2015). Properties of Liquids In Visionlearning. Retrieved February 20, 2016 from http:// www.visionlearning.com/en/library/Chemistry/1//222/reading A. LESSON PRELIMINARIES 1. Prayer 2. Checking of Attendance 3. Health protocols 4. Presentation of objectives and unlocking of difficult terms ELICIT (5 mins) Note: (Managed learner behavior constructively by applying positive and non-violent ensure discipline to learning-focused environments) Note: From the beginning of the discussion teacher will remind the learners that during recitation their thoughts in mind, personal point of views and ideas are wellappreciated. (Planned implemented managed and developmentally sequence teaching and learning processes to meet curriculum requirements and varied teaching contexts.) During the conduct of classroom orientation. Students were already assigned to their respective groups and the class was divided into four groups. Every session leader was tasked to report the attendance of his/her members. Prior to the start of formal classes, teacher identified learners who are suffering from visual problems (myopia) and were assigned to seat in front of the class. B. REVIEW OF THE PREVIOUS LESSON (applied knowledge content within the curriculum areas) -To be conducted Individually (Call students who will answer the given questions) Process question: 1. Based on your Grade 9 lesson, what is covalent bonding? 2. What is ionic bonding? 3. Explain how ions are formed? UNLOCKING DIFFICULT TERMS: a. Electronegativity d. Covalent bond b. Valence electron e. Miscible c. Dipole f. Immiscible Note: To meet the content of WITHIN the curriculum areas teacher will recall their previous lesson in Grade 9, second quarter particularly lesson 1 about ionic and covalent bonding and how ions are formed. Learners will explain their knowledge regarding the previous lesson, whereas the teacher may give her clarification about the notions given by the learners. Difficult terms will be unlocked by students posting the different definitions written on strips of paper placed below random chairs. CLASSROOM OBSERVATION TOOL1 P a g e 2|9 Note: DLP execution following its content, processes, and strategies. C. PRESENTING THE NEW LESSON (applied knowledge content across the curriculum areas) -To be conducted Individually (Call students who will answer the given question) Process question: 1. How do you think does the food processing industry make use of the properties of different compounds found in different ingredients to create// cook sumptuous meals in five-star restaurants? Note: To meet the content of ACROSS the curriculum areas teacher will relate the chemical properties of different chemical compounds found specifically in the ingredients for making bread and pastry and those of different condiments and spices for cooking meals in restaurants. TLE_HEBP9- 12PP-IIa-g-4, TLE_AFFP9- 12NB IIIf-j-2 Clarify to the students that the current lesson relates to their previous subject in Grade 9 Science by explaining its connection during the discussion. S9MT-IIa-13, S9MT-IIb-14, S9MT-IIe-f-16 B. Establishing a purpose for the lesson Activity #1: POLARITY EXPERIMENT (managed classroom structure to engage learners, individually or groups in meaningful exploration, discovery, and hands-on activities within a range of physical learning environments) - Experiment to be conducted by group. ENGAGE (10mins) Materials: water, vinegar, vegetable oil, gasoline, food coloring (optional for coloring water and vinegar), test tubes or graduated cylinder, stirring rod. (Managed classroom structure to engage learners, individually or groups in meaningful exploration, discovery, and hands-on activities within a range of physical learning environments) Procedure: Mix the following samples well with a spoon and observe their reactions. Stir the mixture. Remember to wash and dry the stirring rod after each use. a. Water + vinegar b. Water + oil c. Water + gasoline d. Oil + vinegar e. Oil + gasoline Note: Teacher will give Disposal: activity that allow learners to Samples with oil and gasoline should first be mixed with dishwashing liquid before disposing down the sink. work collaboratively by sharing their own ideas with their groupmates. Have the learners hypothesize why certain combinations mix better than others. Ask them to group the samples together according to how well they mix. Learners should be able to predict the results of the experiment as they mix oil and water. They should recall simple chores they do at home such as washing out oil from pans. Suggest that water and vinegar can be grouped together while oil and gasoline belong to another group. These substances can be classified as polar and non-polar substances. The difference in polarity explains why certain combinations mix and not others. (Applied knowledge content across the curriculum areas) Note: To meet the content of ACROSS the curriculum areas teacher give examples of instances in food prcessing and bread and pastry making where the polarity of molecules apply and explain what will happen to food cooked if the ingredients used were not mixed properly due to factors of polarity. The learners will explain their answers in front of their classmates thru recitation process. Hence, the teacher will give the correct answer and help the learners understand better the lesson. CLASSROOM OBSERVATION TOOL1 P a g e 3|9 Presenting example instances of the new lesson. EXPLORE (20 mins) CLASS DISCUSSION The teacher will introduce the two factors that determine the polarity of molecules, these are: 1) The polarity of the bonds between atoms which can be studied based on electronegativity, and 2) The geometrical shape of the molecule which can be predicted via the valence shell electron pair repulsion (VSEPR) theory. - Bond Polarity (20 minutes). (Used a range teaching strategy that enhance learner achievement in literacy and numeracy skills.) Note: Allow learners to think critically by answering process questions that is not answerable by yes or no only based on the experiment done Review Grade 9 chemistry discussions on properties of elements found in the periodic table such as boiling points, melting points, oxidation number, etc. Point out that one of the properties found in the periodic table is the electronegativity of elements. Electronegativity (EN) - Measure of the relative tendency of an atom to attract electrons to itself when chemically combined with another atom. The higher the value of electronegativity, the more it tends to attract electrons toward itself. Introduce polar covalent and non-polar covalent bonds. Polar covalent bonds occur when electron pairs are unequally shared. The difference in electronegativity between atoms is significant. Examples of compounds having polar covalent bonds are: HCl EN of H = 2.1 EN of Cl = 3.0 ΔEN = 0.9 HF EN of H = 2.1 EN of F = 4.0 ΔEN = 1.9 The separation of charges makes the bond polar. It creates an electric dipole. Dipole refers to “two poles,” meaning there is a positive and a negative pole within a molecule. Elements with the higher EN value become the partial negative pole while elements with the lower EN value become the partial positive pole. This makes the molecule a polar molecule Non-polar covalent bonds occur when electron pairs are shared equally or the difference in electronegativity between atoms is less than 0.5. Examples of substances having non-polar covalent bonds are: H2 EN of H = 2.1 ΔEN = 0.0 NON-POLAR MOLECULE; not a dipole Cl2 EN of Cl = 3.0 ΔEN = 0.0 NON-POLAR MOLECULE; not a dipole F2 EN of F = 4.0 ΔEN = 0.0 NON-POLAR MOLECULE; not a dipole HI EN of H = 2.1 EN of I = 2.5 ΔEN = 0.4 POLAR MOLECULE; a dipole Provide several examples and have the learners determine if the bond between elements are polar covalent or non-polar covalent. Seatwork CH4 CF4 O2 HBr Answer key CH4 EN of H = 2.1 EN of C = 2.5 ΔEN = 0.4 Non polar covalent bond CF4 EN of C = 2.5 EN of F = 4.0 ΔEN = 1.5 Polar covalent bond O2 EN of O = 3.4 ΔEN = 0.0 Non polar covalent bond HBr EN of H= 2.1 EN of Br = 2.8 ΔEN = 0.7 Polar covalent bond Proceed to discuss molecular geometry, another important factor which determines if a molecule is polar or not. CLASSROOM OBSERVATION TOOL1 P a g e 4|9 Discussing new concepts and practicing new skills # 1. EXPLAIN (30 mins) (Applied a range of teaching strategies to develop critical The teacher will discuss the next concept. (applied a range of teaching strategies to develop critical thinking and creative thinking as well as higher order thinking skills) - Molecular Geometry (30 minutes) The valence shell electron pair repulsion theory or VSEPR theory helps predict the spatial arrangement of atoms in a polyatomic molecule. The shapes are designed to minimize the repulsion within a molecule. Teacher will show a five-minute video showing the five different geometric shapes under the VSEPR theory, namely, linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. The video also explains the basic principle behind the VSEPR theory. thinking and creative thinking as OldSite Vanden Bout. (2011). VSEPR Theory: Introduction. Retrieved from Note: Allow learners to think https://www.youtube.com/watch?v=keHS-CASZfc well as higher order thinking skills) critically by answering process questions that is not answerable by yes or no only based on the experiment done Present the images below summarizing the different shapes under the VSEPR theory CLASSROOM OBSERVATION TOOL1 P a g e 5|9 Figure 1. Linear, Retrieved May 10, 2016 from http://chemlab.truman.edu/CHEM131Labs/MolecularModeling1.asp Figure 2. Bent, Retrieved May 10, 2016 from https://quizlet.com/4023155/chemistry-molecular-bonding-structure-chs-8-9-flash-cards/ Figure 3. Trigonal planar, Retrieved May 10, 2016 from http://chemlab.truman.edu/CHEM131Labs/MolecularModeling1.asp Figure 4. Tetrahedral, Retrieved May 10, 2016 from http://chemlab.truman.edu/CHEM131Labs/MolecularModeling1.asp Figure 5. Trigonal bipyramidal, Retrieved May 10, 2016 from https://www.studyblue.com/notes/note/n/molecular-geometry-/deck/13026512 Figure 6. Octahedral, Retrieved May 10, 2016 from Trigonal planar and Octahedral http://chemlab.truman.edu/CHEM131Labs/MolecularModeling1.asp Teacher will focus on the basic shapes such as linear, bent, tetrahedral, trigonal pyramidal, trigonal planar, and octahedral. Teacher will emphasize that symmetry plays an important role in determining the polarity of a molecule. Give the following guidelines to determine the VSEPR shape of a molecule: 1. 2. 3. 4. 5. 6. Determine the central atom of a molecule. The central atom is the least electronegative element. Count how many valence electrons the central atom has. Count how many valence electrons the side atoms have. Create the appropriate Lewis structure of the molecule. Using the Lewis structure as a guide, determine the appropriate VSEPR shape for the molecule. Note how many electrons are shared and unshared. This will help determine the appropriate VSEPR shape. Learners will be asked to practice on how to determine and draw different molecular shapes using the examples below. Emphasize that lone pairs have a big factor in making a molecule polar. Polar molecule: H2O Bent - polar due to two lone pairs NH3 Trigonal pyramidal - polar due to one lone pair NO Linear - polar due to unequal sharing of electrons Due to the two lone pairs, the water molecule has a bent shape. Figure 7. Lewis Structures and the Shapes of Molecules, Retrieved February 18, 2016 from https://www.angelo.edu/faculty/kboudrea/general/shapes/00_lewis.htm#PolarNon-polar CLASSROOM OBSERVATION TOOL1 P a g e 6|9 Discussing new concepts and practicing new skills #2. Teacher will mention that one of the most practical manifestations of polarity is solubility and miscibility. Solubility refers to the ability of a solute to dissolve in a certain amount of solvent. Miscibility is the ability of two liquids to mix in all proportions. Solubility, Miscibility, and Polarity Note: Teacher will emphasize different samples when polarity is exhibited and applied in our day to day activities. Developing mastery (Leads to Formative Assessment 3) Teacher will give the general rule that “like dissolves like” or “like mixes with like.” This refers to substances being able to mix due to their same polarity. In the experiment, water and vinegar mixed because they are both polar substances while gasoline and oil are non-polar substances. Oil and water, oil and vinegar, gasoline and water, and vinegar and gasoline do not mix because their polarities are different. PRACTICE SEATWORK (20 MINS) Electronegativity and molecular geometry ELABORATE (5 mins) Determine the polarity of the following compounds based on electronegativity differences and molecular geometry. (Designed selected organized and Molecular geometry used diagnostic formative and Polarity summative assessment strategies 1. HBr 2. PH3 requirements.) 3. SiS2 Note: Teacher will provide a 4. O2 practice seatwork to check the learnings of the student from 5. BCl3 consistent with curriculum linear trigonal pyramidal linear linear trigonal planar polar polar non-polar non-polar non-polar the lesson. Finding practical application of concepts and skills in daily living. (Use differentiated, developmentally appropriate learning experiences to address learners’ gender, needs, strengths, interests, and experiences.) Making generalization and abstraction about the lessons. (5 mins) Evaluating learning REFLECTION (5 minutes) Guide questions: Ask the students to briefly answer the following questions regarding the structure and properties of polar molecules based on their understanding of the topics discussed. 1. Which substances available in your home are miscible in water? Explain. 2. Classify ten substances/compounds present in your surrounding as to their polarity? Group these as to whether it’s used by men/women in the house. How will you explain to your parents if they have been using materials/chemicals the wrong way in relation to its polarity? 3. Relate the polarity of the listed substances and compounds to their properties? 4. How did your understanding of polarity and its property change your perception of different substances and compounds available around you? Note: The learner may give their own ideas about the questions given by the teacher. Whereas the teacher will add some important matters for better understanding. Ask at-least three Students to generalize the proceedings of the lesson. Note: 1. The teacher may also add important information from the lesson based from the answers of the students. EVALUATION (25 MINS) The teacher will give the learners a formative assessment. Determine the following: A. Molecular geometry CLASSROOM OBSERVATION TOOL1 P a g e 7|9 (Designed selected organized and used diagnostic formative and B. Bond polarity between atoms C. Polarity of the molecule summative assessment strategies consistent with curriculum Students should have their periodic tables with them for the exam. requirements.) Bond Polarity Molecular Geometry Polarity of Molecule a. H2O polar bent polar activity 2, evaluation and b. CCl4 non-polar tetrahedral non-polar additional activities. to check the learnings of the student c. BF3 non-polar trigonal planar non-polar from the lesson. d. SF6 non-polar octahedral non-polar EVALUATE e. SiF4 polar tetrahedral non- polar (25mins) Key to correction are highlighted with cerulean color. Check Your Knowledge Additional activities for DIRECTIONS: Read each question carefully. Choose the letter of the best answer. application or Write your answer on a separate sheet of paper. remediation. (Designed selected organized and ____1. Which of the following statement is TRUE about water? used diagnostic formative and a. It is a polar molecule summative assessment strategies b. It is a non-polar molecule consistent with curriculum c. It is both polar & non-polar d. It has no polarity requirements.) ____2. Which of the following will be the solvent if a non-polar substance dissolves in Note: Teacher will provide an unknown liquid? activity 2, evaluation and additional activities. to check a. Non-polar the learnings of the students b. Polar from the lesson. c. Water d. All of the above ____3. Which of the following shapes is most likely form of a non-polar molecule? a. Asymmetric linear b. Bent c. Square planar d. Pyramidal ____4. Which of the following is an example of a non-polar molecule? (Selected developed, organized, a. CO2 and use appropriate teaching and b. H2O learning resources, including ICT. c. NH3 to address the learning goals.) d. SO2 Note: For take home activity ____5. Which of the following is an example of a polar molecule? learners will research five other a. HCl samples of the following arts b. BF3 given by the teacher to utilize c. CCl4 the use of ICT they will attach picture from different websites d. XeF4 Note: Teacher will provide and print the hardcopy of the activity. It enables them also to explore their knowledge on the use of Microsoft word. EXTEND KEY TO CORRECTION 1. B 2. B 3. D 4. A 5. B Assignment Directions: Complete the table below, based on your understanding of the polarity of molecules, their structure, and their properties. CLASSROOM OBSERVATION TOOL1 P a g e 8|9 Key to Correction: V. REMARKS VI. A. REFLECTION No. of learner who earned 75% in the evaluation B. No. of learners who require additional activities for remediation who scored below 75% C. Did the remedial lesson work? No. of learners who caught up with the lesson D. No. of learners who continue to require remediation E. Which of my teaching strategies worked well? Why did this work? F. What difficulties did I encounter which my principal or supervisor can help me solve? G. What innovation or use/discover which I wish to share with other teachers? Prepared and demonstrated by: OLGA VICTORIA B. CEDIÑO, LPT, JD. Teacher- II Checked and observed by: JENNIFER V. GARCIA STEM Coordinator / MT-II Noted: EDWIN G. RETURAN Assistant Principal II - SHS CLASSROOM OBSERVATION TOOL1 P a g e 9|9