1.3 Review Name________________________________ Period________Date____________________
advertisement

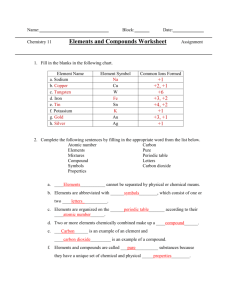
1.3 Review Name________________________________ Mixtures, Compounds and Elements Period________Date____________________ Identify each of the following as heterogeneous, homogeneous (mixture), or pure substance: _____________________________ 1. alphabet soup ___________________________ 6. paint _____________________________ 2. table salt ___________________________ 7. sea water _____________________________ 3. concrete ___________________________ 8. granite _____________________________ 4. water ___________________________ 9. steel _____________________________ 5. air ___________________________ 10. sugar Completion: Complete the following sentences using the correct separating technique term. Some terms may be used more than once. filtration crystallization (evaporation) chromatography electrolysis distillation 11. Heterogeneous mixtures are often separated by __________________________. 12. Separating sand from water can be done by ___________________________. 13. The sugar in sugar water can be removed by ___________________________. 14. The separation technique that takes advantage of different boiling points is called ________________. 15. Removing chlorophyll pigment from leaves might be done by ______________________________. 16. The best way to decompose water into hydrogen and oxygen is by ____________________________. 17. Crude oil is broken down by heat, vaporized, and allowed to condense into various liquids such as gasoline. This process is called __________________________. Short answer: 18. How could you separate a mixture of iron fillings and aluminum fillings? What property of these metals would allow such a separation? Identify each of the following materials as either an element, (E), or a compound (C): ______ 19. carbon ______ 23. plastic ______ 27. silicon dioxide ______ 20. water ______ 24. tin ______ 28. carbon dioxide ______ 21. aluminum foil ______ 25. arsenic ______ 29. helium ______ 22. sodium chloride ______ 26. ethanol ______ 30. methanol Fill in the blank portions of the chart: Element Name Element Symbol Derivative Name 30. Na natrium 31. Cu cuprum Lead 32. plumbum 33. W wolfram 34. Fe ferrum 35. Sn stannium 36. Potassium Au 37. 38. aurum Mercury hydrargyrum Ag 39. Completion: elements properties kalium symbols letters periodic table compound argentum mixtures pure carbon dioxide 40. ______________________ cannot be separated by physical or chemical means. 41. Elements abbreviated with ___________________, which consist of one or two _________________. 42. Elements are organized on the ________________________________. 43. Two or more elements chemically combined make up a _______________________. 44. Carbon is an example of an element, and _______________________ is an example of a compound. 45. Elements and compounds are called __________________ substances because they have a unique set of chemical and physical _________________________. 46. List elements that were named for famous scientists: #96 ___________________#99______________ #100________________________#101__________________________ #102 ___________________ #103 _______________________#104__________________________ #106 ___________________ #107 _______________________#109__________________________ #111 ___________________ 47. Two substances were tested and were found to have the following chemical compositions. Substance A Substance B 73% oxygen 27% carbon 57% oxygen 43% carbon Are these substances the same compound? Explain. _______________________________________ _________________________________________________________________________________