Thermodynamics
advertisement

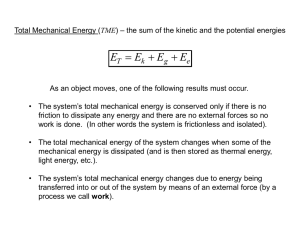
Thermodynamics Phase Change • Three states of matter (Solid, liquid, gas) • With increase in thermal energy a substance can – Increase temp or – Change phase • Draw phase change diagram • Evaporation: – Adding energy to a liquid until a phase change to gas. • Condensation: – Humidity is the amount of water vapor contained in the air. Saturation is the point when the air cannot hold any more water. • Sublimation/ dissolution – Changing phase by skipping liquid phase. Zeroth Law • When two objects of dissimilar temp come into contract heat transfer occurs. 1st Law • When heat flows from a system, the system gains or loses an amount of energy equal to the amount transferred. • Heat Added = increase in Internal Energy + Work • Gas-Piston-Cylinder – Adiabatic system. No heat is lost to the enviroment. 2nd Law • Heat of itself never flows from a cold object to a hot object. • Work is required to make heaters and AC work. (example: opening a C02 cylinder) • Perpetual motion of the 2nd kind. 3rd Law • There is no temperature below Absolute Zero. Efficacy • Efficacy defines how much work was done. • Describe a 4 stroke combustion engine. Entropy – a measure of disorder • • • • Order tends to become disorder. Higher Quality energy (i.e. Chemical). useful energy turns in thermal energy. Thermal energy is dissipated across the universe.