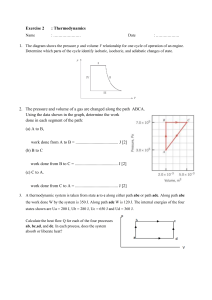
HEAT, WORK AND INTERNAL ENERGY GLOBAL WARMING? THERMODYNAMICS: the science of energy, specifically heat and work, and how the transfer of energy effects the properties of materials. Thermodynamics: "thermo": Greek therme heat "dynamics": Greek dynamikos powerful Physics that deals with the mechanical action or relations between heat and work Example 1: Heat to work Heat Q from flame provides energy to do work --------------------------------------------------------Example 2: Work to heat. Work done by person is converted to heat energy via friction. A “system” is the “collection of objects on which attention is being focused” The “surroundings” are everything else in the environment The system and surroundings must be separated by walls which can either insulate or allow heat flow OPEN SYSTEM: Mass and energy freely moves in and out between the system and the surrounding ISOLATED SYSTEM: No interaction between the system and the surrounding CLOSED SYSTEM: fixed mass Heat, Q, energy caused by temperature difference Heat ... is the amount of internal energy entering or leaving a system ... occurs by conduction, convection, or radiation. ... causes a substance's temperature to change ... is not the same as the internal energy of a substance ... is positive if thermal energy flows into the substance ... is negative if thermal energy flows out of the substance ... is measured in joules Thermal Equilibrium Systems (or objects) are said to be in thermal equilibrium if there is no net flow of thermal energy from one to the other. A thermometer is in thermal equilibrium with the medium whose temperature it measures, for example. If two objects are in thermal equilibrium, they are at the same temperature. Work, W, energy caused by physical motion WORK W is positive if work is done by system. W is negative if work is done on the system. Air does work on the environment: W > 0. Environment (man) does work on system: W < 0 (Alternative: system does negative work because force by air pressure on thumb is opposite to the direction of motion of the thumb.) RELATIONSHIP BETWEEN HEAT AND WORK Why does the volume of gas expands when it is heated? W=Fxd Pressure (P) = (Force) F or F=PA (Area) A Volume (V) = L x W x H or A x d d=V A W=PAV =PV A Internal Energy (U or E) : (measured in joules) - Sum of random translational, rotational, and vibrational kinetic energies DU: change in U DU > 0 is a gain of internal energy DU < 0 is a loss of internal energy ---------------------------------Thermal Energy: same as internal energy Vibrational kinetic energy in solids. The hotter the object, the larger the vibrational kinetic energy Motions of a diatomic molecule in a fluid INTERNAL ENERGY (U or E) is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with the vibrational and electric energy of atoms within molecules or crystals. The First Law of Thermodynamics states that : The internal energy of a system changes from an initial value Ui to a final value Uf due to heat added (Q) and work done by the system (W) DU = Uf – Ui = Q – W Q is positive when the system gains heat, and negative when the system loses heat. W is positive when it is done BY the system, and negative when it is done ON the system Example: 1000 J of thermal energy flows into a system (Q = 1000 J). At the same time, 400 J of work is done by the system (W = 400 J). What is the change in the system's internal energy U? ---------------------------------------------------------- Solution: DU = Q - W = 1000 J - 400 J = 600 J Example: 800 J of work is done on a system (W = -800 J) as 500 J of thermal energy is removed from the system (Q = -500 J). What is the change in the system's internal energy U? ----------------------------------------------------- Solution: DU = Q - W = -500 J - (-800 J) = -500 J + 800 J = 300 J Work Done by an Expanding Gas W = PDV DV = Vf - Vi W = P (Vf - Vi) Area under pressure-volume curve is the work done ----------------------------------------Isobaric Process: "same pressure" Greek: barys, heavy Work and the Pressure-Volume Curve Work Done = Area Under PV curve ------------------------------------How much work is done by the system when the system is taken from: (a) A to B (900 J) (b) B to C (0 J) (c) C to A (-1500 J) ------------------------------------Each "rectangle" has an area of 100 Pa-m3 = 100 (N/m2)-m3 = 100 N-m = 100 Joules Expanding Gas 10 grams of steam at 100oC at constant pressure rises to 110oC: P = 4 x 105 Pa DT = 10oC DV = 30.0 x 10-6 m3 c = 2.01 J/g oC Example: If a gas expands at a constant pressure, the work done by the gas is: W = PDV What is the change in internal energy? DU = Q - W W = (4 x 105)(30.0 x 10-6) = 12 J Q = mcDT = (10)(2.01)(10) = 201 J DU = Q - W = 201 J - 12 J = 189 J Work, Rubber Bands, and Internal Energy DU = Q - W Expand rubber band: W < 0, Q = 0 DU >0 temperature increases ------------------------------------------Press thick rubber band to forehead and expand it rapidly. The warming should be obvious. Now allow the band to contract quickly; cooling will also be evident. ISOTHERMAL-Temperature remains constant ISOBARIC - Pressure remains constant ISOMETRIC - Volume remains constant (also ISOVOLUMETRIC or ISOCHORIC) Since ΔV = 0, W = 0 then DU = Q - W = Q Adiabatic Expansion of a Ideal Gas No heat transfer therefore no temperature change (Q=0). Generally obtained by surrounding the entire system with a strongly insulating material or by carrying out the process so quickly that there is no time for a significant heat transfer to take place. If Q = 0 then ΔU = - W A system that expands under adiabatic conditions does positive work, so the internal energy decreases. A system that contracts under adiabatic conditions does negative work, so the internal energy increases. Adiabatic Expansion of a Ideal Gas Both adiabatic expansion and compression of gases occur in only hundredths of a second in the cylinders of a car’s engine. Blowing air through wide open mouth results to warm air. Blowing through small opening results to cooler air due to adiabatic expansion. Compresses air leaking out through a small opening also results in adiabatic cooling. PROCESS DIAGRAMS: visualize processes using properties (T, P, V, etc.) Area underneath the slope represents the amount of work done (P x V). CYCLE: a system undergoes processes returning to its initial state Area underneath the slope represents the amount of work done (P x V). Refrigerators work by taking heat from the interior and depositing it on the exterior The compressor raises the pressure and temperature of the refrigerant (freon or ammonia) while the coils OUTSIDE the refrigerator allow the now hot refrigerant to dissipate the heat The warm refrigerant flows through an expansion valve from a high-pressure to a low-pressure zone, so it expands and evaporates • The coils INSIDE the refrigerator allow the cold refrigerant to absorb heat, cooling the interior • The cool refrigerant flows back to the compressor, and the cycle repeats Second Law of Thermodynamics Heat flows naturally from a region at high temperature to a region at low temperature. By itself, heat will not flow from a cold to a hot body. When an isolated system undergoes a change, passing from one state to another, it will do so in such a way that its entropy (disorder) will increase, or at best remain the same. ENTROPY Can you beat the Second Law? So, can you cool your kitchen by leaving the refrigerator door open NO! The heat removed from the interior of the refrigerator is deposited back into the kitchen by the coils on the back! And to make matters worse, the Second Law of Thermodynamics says that work is needed to move the heat from cold to hot, so the actual amount of heat added to the kitchen is MORE than the amount removed from the refrigerator Hopefully, you understand today’s lesson. Otherwise, you’ll end up like this cow.