Guided Reading Chapter 10 Section 1 and 2 molecules?

Guided Reading Chapter 10 Section 1 and 2
1. What is Brownian motion? How does it provide evidence that matter is made of atoms and molecules?
Irregular, jerky motion of particles; all tiny particles are moving.
2. Matter is anything that has mass and takes up space.
3. A(n) Element is the smallest bit of matter that is pure and can’t be broken down into any other smaller substance by physical or chemical means.
4. A(n) Atom is the single smallest particle of an element that retains the chemical identity
5. A compound is a substance that contains Two or more different elements joined chemically, and has the same composition throughout.
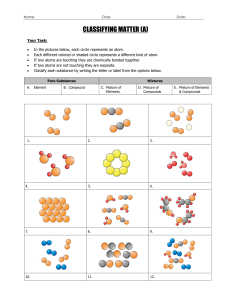
6. Sketch the picture of an element, compound, and mixture at the bottom of page 230.
Element Compound Mixture
7. Using words and looking at the diagram from pg 230 describe how elements, compounds and mixtures are different from each other.
Element – only 1 kind of atom
Compound – 2 or more atoms, 1 type of molecule
Mixture – any combination of 2 or more elements and/or compounds (physically combined)
8. The three units used to measure temperature are Celsius , Fahrenheit and Kelvin (last one is found on pg 238).
9. Thermal Energy is caused by the back and forth “jiggling” of molecules.
10. A Thermometer is an instrument that measures the exact temperature.
11. Digital thermometers work by measuring a change in Electrical Resistance which changes with a change in temperature.
12. While there is no upper limit to temperature there is a lower limit called Absolute Zero , It is the temperature at which molecules Cease moving entirely .
Vocabulary Chapter 10 Section 1 and 2
1. Matter is anything that has mass and takes up space.
2. A pure substance that cannot be broken down into simpler substances by physical or chemical means is a(n) Element
3. The smallest particle of an element is a(n) Atom
4. A(n) Compound is a substance that contains two or more elements that are
5. A(n) Molecule is a group of two or more atoms joined together by chemical bonds.
6. A(n) Pure Substance cannot be separated into other types of matter by physical means.
7. Matter that contains a combination of different elements and/or compounds and can be separated by physical means is called a(n) Mixture
8. A(n) Homogeneous is a mixture that is the same throughout.
9. A(n) Heterogeneous is a mixture that is not the same throughout.
10. Temperature is the measure of the average kinetic energy of the particles in matter
11. Farenheit is a temperature scale in which water freezes at 32 degrees. This is used in
12. Celsius is a scale in which water freezes at 0 degrees. This is used in science.
13. Energy due to temperature is called Thermal Energy
14. You measure temperature with a(n) Thermometer
15. The lowest possible temperature is called Absolute Zero
16. The Kelvin is a temperature scale that starts with absolute zero.