R & M A N

R & M A N S P E C T R U M O F C R Y S T A L L I N E
A M I N O S U L P H O N I C A C I D
BY N. IOUSHN~~:RTHY
(Department of Physic~,
Indian Institute of Science, Bangalore-12)
Rr162 Novembr 30, 1964
(Communicated by Prof. R. S. ~ , I.A.sr
1. INTRODUCTION
AMINOSUL1DHONIC ACID or sulphamic acid NHs + SO3- is the simplest defivative of sulphufic acid and has been known to be ah interesting compound from the point of view of spectroscopic and structural studies (Vuagnant and
Wagner, 1957; Gupta and Majumdar, 1941). At present, only an incomplete study of its Raman spectrum by Gupta and Majumdar (1941) is available.
As large single crystals of sulpham~c acid can be easily grown from aqueous solution, it was thought desirable to reinvestigate its Raman speetrum and to assess the nature and symmetry of these molecules in the crystal. The more detailed study of the infra-red absorption spectrum of sulphamic aeid over a wide range of temperatures by Vuagnant and Wagner (1957) has been used in the assignment of the observed frequencies.
2. EXPERIMENTAL DETAILS
Single crystals of sulphamic acid exhibiting well-defmed faees eould be easily grown from aqueous solutions of the pure substance. The biggest erystal grown had roughly the size 2 0 • 2 1 5 As the crystals were found to be transparent to the ultra-violet, the resonance radiation A 2537 of mereury was used for the excitation of the Raman spectra. Prelimiuary experiments revealed the presence of very closely spaeed lattiee lines and therefore in the final intense spectra a ¡ slit of 0-015 mm. was used. With the Hilger medium quartz spectrograph and Zenith Astronomical plates, exposures of about six hours were found to be necessary to record intense spectra.
3. RESULTS
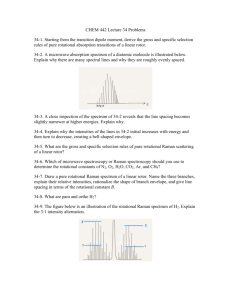
Figure 1 b i s ah enlargement of the Raman spectrum of a single erystal of sulphamic acid with its microphotometer record in Fig. 1 c. Figure I a
I46
Raman Spectrum of Crystalline Aminosulphonic Acid
147 is the eomparison mereury spectrum in the ultra-violet region. The posifions and frequency shifts of the Raman lines are marked on the micro- photometer record. They are also listed in Table I. The figures given in brackets represent the relative lntensities of the Raman lines estimated visually. Thirty-eight distinct Raman lines have been recorded. Of these, the first seven belong to the lattice spectrum while the remaining ones arise from the internal osciUations. The infra-red data are also included in the same table. There is good agreement between the Raman and infra-red frequencies in the region investigated.
4. DISCUS$ION
Crystal structure data.--The
crystal structure of sulphamic acid was determined by Kanda and King (1951) and by Osaki, Tadokoro and Nitta
(1955) who found that sulphamic acid crystallises in the orthorhombic system with the space group Vh ~ 5 - Pbca. The unit cell dimensions a r e a = 8" 115
-4- 0"001 A, b = 8"0664- 0.001A and c----- 9.2554- 0.003A. There are eight molecules in the unit cell. The acid crystallises as Zwitter ion molecules
NH3 + SO3- of a distorted tetrahedral f6rm with the existence of extensive hydrogen bonding of the type N + - - H . . . . O with each NHa + group entering into five bridging systems. The hydrogen bond distances vary from 2.82 A to 3.07 A, which has been confirmed by neutron diffraction studies by Sass
(1960). The ¡ hydrogen bond distances are 2.82 A, 2.83 A, 2.94 A,
3"02 A and 3-07 A.
External oscillations.---The
conventional group theoretical methods of
Bhagavantam and Venkatarayudu (1948) can be applied to ¡ the ( 3 p - 6) internal molecular modes each q fold degenerate and 3 q lattice rocking and
(3 q -- 3) lattice translational modes of a unit cell with q, p-atomic molecules.
Suela a ealculation was performed treating the Zwitter ion a s a unit and showed that twelve rotatory type and twelve translatory type of lattice oscillations can be expected to appear in Raman effect. The discrepancy between the observed and the calculated number of latfice lines appears to be due to the overcrowding of all these lines in a region of smaU frequency shifts. On account of their high intensities, the lines at 110, 120, 140 and 158 cm. -1 can be attributed to rotatory type of lattice oscillations. The lines at 170 and
240 cm. -x stand out clearly from the rest of the lattice lines in their breadth and structure even though their intensities are different. These two lines have been attributed to the vibrations of the hydrogen bonds N H - - O . Similar low-frequency shifts due to hydrogen bond oscillations have been observed by Gross (1959) and Balasubramanian and Krishnan (1958).
148 N.K.~Sa'N~~.rR~-XY
TABLE I
Vibration spectrum of sulphamic acid (frequency shifts in cm. -~)
SI. No.
Raman effect present
Infra-red
Vuagnant and Wagner
Assi.~mment
19
2O
21
22
23
24
25
26
27
28
9
10
11
12
13
14
15
16
17
18
1
2
3
4
5
6
7
$
29
30
31
32
33
34
35
36
37
38
50 ( 2 )
68 ( 1 )
80 ( 3 )
110 ( 3 )
125 ( 4 )
1 4 0 ( 4 )
155 ( 4 )
170 ( 2 )
240 ( 5 )
357 (10) }
378 (10)
535 (10) l
5 5 0 ( l O ) ,
678 (10)
697 (10)
862 ( ? )
9 0 5 ( 5 )
1010(--) •
1020 ( 8 ) )
1057 (20)
1080 (10)
1265 (10)
1280 (1(3)
1302 ( 8 ) )
1319 (10)
1440 ( 6 )
1 5 3 4 ( 5 ) }
1571 ( 8 )
2460 ( 5 )
2476 ( 4 )
2539 ( 3 )
2553 ( 2 )
2579 ( 3 )
2876 ( 8 )
2906 ( 2 )
3050 ( 5 )
3142 ( 5 )
3260 to
3450
..
. .
..
..
..
...
~i~
.,.
1,#t6
1 5 4 2 }
1570
1786
2044
2152
2345
2465
...
_.
25 91
2893
Lattiee
,,
,,
,,
,,
,,
NIŸ '
. . . . O
N H . . . . O
352
526~
540), vn (e)
,,~, ( e )
682
695
.
.
.
.
.
uj(ax)
_ . .
I000~5}
1064
...
1262 i312 v)
(e) v, (aD v a ia~)
..
,,, (e) v, iax) v, (e)
3()55
3140
... v~ (al) + v)(e)* v~ (al) + v)(e)* v~ (e) + v 9 (e)-* v~ (e) -+- v, (e)* v, (e) + v, (e)*
N - - H . . . . O bonded
N - - H . ' . ' . . O-bonded
N - - H . . . . O bonded
Band
* Combinations with the various components into which the degenerate mode is split Ult.
Raman Spectrum of Crystalline Aminosulphonic Acid
149
Infernal oscillations.mThe
free sulphamic acid molecule exists a s a Zwitter ion having Csv symmetry and hence its fundamental vibrations are distributed under the different symmetry types as below: 5 a, + a2 + 6 e. The torsional vibration about the N - - S bond (vlz) belonging to the al type is forbidden to appear in the vibrational spectrum. AU the remaining vibrations are active in both Raman effect and infra-red absorption. The vibrational assignments of the vadous modes of the free sulphamic acid a r e a s follows: vi (al)--symmetric stretching NH + v i (ax)--symmetric deformation NH3 + vs (a0--symmetrie stretehing SOz- v~ (ax)--symmet¡ deformation SO~ v a (a~)gN--S stretching v 6 (e)--degenerate stretching NH3 + v~ (e)~degenerate deformation NH3 + vs(e)----degenerate stretching SO3- v i (e)--degenerate rocking NH3 + v~0 (e)--degenerate deformation SOs- v n (e)--degenerate rocking SOz- vxl ( a 0 - - N - - S torsion.
But in the crystal, the site symmetry of the Zwitter ion is Cx and h e n e e the degeneracies of the doubly degenerate modes are removed and also the torsional mode becomes active. Assuming that the site symmetry is not far from Cs due to the coupling of the modes of the eight moleeules in the unir eell, one should have a total of one hundred and forty-four modes distributed among the various species "as follows: 22 A~ a + 22 Blg + 14 Blg + 14 Bag
+ 14 Axn + 14 Blu + 22 Bl,, + 22 Bau wherein the first four species are
Raman active and the last three are infra-red active. The modes eoming under Axn are forbidden in both. Since the actual site symmetry is ex and there ate eight molecules in the unit cell each mode coming under the species
A' and
A"
split into eight modes corresponding to aH the eight species of the point group of the crystal and we arrive at the result that in the crystal one can expect 54 fundamental components in infra-red absorption and 72 in
Raman effect. are
In the observed Raman spectrum, we find that aU the degr spUt up modes
(see
Table I). The v i (e) mode falls near the mercury line at
2603.20 A and its appearance Ÿ verified by considering the relative intensity of the mercury line. The splitting of the degenerate modes vades from
150 N. I ~ ~ s r n ~ ~ r H Y
15 to 36 era. -1 and indieates that the distortion of the Zwitter ion from the C-~v symmetry is not very large. The coupling of the ruedes may give rise to r spaeed lines and have therefore escaped detection due to the dispersion of the speetrograph.
Ah interesting feature of the observed spectrum is the high intensity of the v~ (a~) symmetrie SO~ deformation vibration at 1057 cm_ 1 It is found to be excited even by the mereury lines )~ 2652.04 ,~, ~ 2653.68/~ and ~ 2655.10 ~, aiad these are marked in the microphotometer record with shifts 2773, 2796 and 2820 cm. -1 The doubly degenerate SOa deformation ruedes en and ex0 appear with considerable intensity on the anti-Stokes side also. In the region of the NH3 + stretching, there are four lines at 2876, 2908,
3050 and 3142 era. -x The latter two are actually maxima in the band while the first is a very strong lŸ Its frequency shift is consistent with the re- ported N - - H . . . . O hydrogen bond length of 2.82 A while the second may be the N - - H . . . . O bonded oscillation corresponding to the N - - H . . . . O dis- tanec of 2.83 A. The 3050 cm. -~ line can be assigned to the hydrogen-bonded vibration for N - - H . . . . O distante o f 2-94 A. These conclusions have been arrived at from the N - - H frequency and N - - H . . . . O distante eurve reported by Krishnan and Krishnan (1964). The five broad and weak lines in the region of 2460-2579 cm. -~ eannot be explained as due to hydrogen bonds of lengths
2.82-3.07 A. and are therefore overtones and combinafions of the ftmda- mentals. The band extending from 3260 to 3450 cm. -x has been assigned as duc to the combinations involving the low frequency N H - - O vibrations and the N - - H . . . . O hydrogen-bonded stretching vibrations. T h e absence of banda corresponding to N H stretching vibrations occurring beyond 3150em.-~ and the smaUer splitting of the degenerate modes conflrm the presenee of
Zwitter ions NI-Is + SOs- and only the slight distortion of the molecular symm•try Csv of the free acid. It will be interesting to study the Raman spee- tra at low temperatures for understanding the related phenomena such es frev rotation and the low-frequency hydrogen bond transitŸ
5. StrMM~Y
The Raman spectrum of a single crystal of sulphamic acid has been recorded with ~ 2537 excitation. Thirty-eight lines have been observed, of which twcnty-nine have been recorded for the first time. Seven Raman lines with shifts in the region 50--155 era. -1 have been assigned to the lattice oscilla- tions, two at 177 and 240 cm. -1 have been attributed to the low-frequeney hydrogen bond vibrations.. The splitting of the degenerate ruedes and the appr of N - - H . . . . O bonded stretching vibrations are comistent with
Raman Spectrum of Crystalline Aminosulphonic Acid
151 the structural data which expect the presence of the free moleeule as a Zwitter ion with only slight distortion from Csv symmetry.
6. ACKNOWLEDGEMENT
The author wishes to express bis heart-felt thanks to Prof. R. S. Kristman for bis suggestions and guidance.
7. REI~ERENCES
1. Bhagtvantam, S. and
Venkatarayudu, T.
Theory o f Groups and Its Applications to Physical Problem91
Andhra University, Waltair, 1948.
2. Gross, E.F. 9 9 Hydrogen Bonding, by Hadzi, Pergamon Press, 1959, p. 203.
3. Gupta, S. J. and Majumdar,
A, K.
J. In. Chem. Soc., 1941, 18, 457.
4. Kanda, F. A. and King, A. J. Jour. Amer. Chem. Soc., 1957, 73, 2315.
5. Krishnan, R. S. and Bala- subramanian, K.
Proc. lnd. Acad. Sci., 1958, 47 A, 55; 1958, 48A, 138. li. ~ and Krishnan, K. ..
7. Osaki, K., Tadokoro, H. and
Nitta, I.
Ibid., 1964, 60A, 11.
Bull. Chem. Soc. (Japan), 1955, 28, 524.
8. Sass, R.L.
9. Vuagnant, A. M. and
Wagner, E. L.
.. Acta Cryst., 1960, 13, 320.
J. Chem. Phys., 1957, 26, 77.
EXPLANATION OF PLATE
XI
FIG. 1.
(a) Mercury spectrum.
(b) Raman spectrum of Sulphamic Acid.
(c) Microphotometer record.