CHEM 442: Molecular Spectroscopy Problems
advertisement

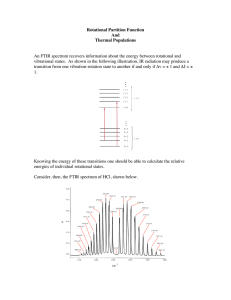
CHEM 442 Lecture 34 Problems 34-1. Starting from the transition dipole moment, derive the gross and specific selection rules of pure rotational absorption transitions of a linear rotor. 34-2. A microwave absorption spectrum of a diatomic molecule is illustrated below. Explain why there are many spectral lines and why they are roughly evenly spaced. 34-3. A close inspection of the spectrum of 34-2 reveals that the line spacing becomes slightly narrower at higher energies. Explain why. 34-4. Explain why the intensities of the lines in 34-2 initial increases with energy and then turn to decrease, creating a bell-shaped envelope. 34-5. What are the gross and specific selection rules of pure rotational Raman scattering of a linear rotor? 34-6. Which of microwave spectroscopy or Raman spectroscopy should you use to determine the rotational constants of N2, O2, H2O, CO2, Ar, and CH4? 34-7. Draw a pure rotational Raman spectrum of a linear rotor. Name the three branches, explain their relative intensities, rationalize the shape of branch envelope, and give line spacing in terms of the rotational constant B. 34-8. What are para and ortho H2? 34-9. The figure below is an illustration of the rotational Raman spectrum of H2. Explain the 3:1 intensity alternation.