The catalytic hydrogenation of certain aliphatic amines by Robert A Currie
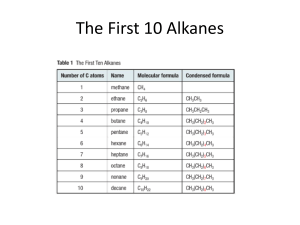
advertisement

The catalytic hydrogenation of certain aliphatic amines
by Robert A Currie
A THESIS Submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree
of Master of Science in Chemical Engineering
Montana State University
© Copyright by Robert A Currie (1960)
Abstract:
The effects of pressure> temperature, and space velocity on the hydrodenitrogenation of certain
aliphatic amines were studied. The feed concentration and hydrogen rate were held constant for all
runs. The compounds studied were n-butyl amine, octyl amine, iso-butyl amine, sec-butyl amine, and
tert-butyl amine. An increase of temperature caused a corresponding increase in the conversion of the
nitrogen compound. An increase in pressure also caused an increase in the nitrogen conversion.
By comparing n-butyl amine to octyl amine, it was evident that the lower molecular weight amine
denitrogenated more readily. Similarly, the isomers of butyl amine denitrogenated in the order n-butyl
amine, iso-butyl amine, sec-butyl amine and tert-butyl amine, with the ease-of denitrogenation
increasing respectively. The product formed from n-butyl amine is a mixture of iso-butane and
n-butane. Sec-butyl amine forms essentially n-butane, while iso- and tert-butyl amine form essentially
iso-butane at high conversions. THE
.CATALYTIC HYDROGENATION
•OF
CERTAIN
ALIPHATIC AMINES
by
ROBERT A. ,CURRIE
O
A THESIS
Submitted to the Graduate Faculty
in
p a r t i a l •f u l f i l l m e n t •of t h e 'requirements
f o r .the degree of
Master:
of Science in Chemical Engineering
at
.Montana State College
Approved:
Head, Major Departm^p
Jean, Graduate Division
Bozeman,. Montana
J u n e ,.i960
'
J
i!.;
fyl'
I A
f
r;:ii;■
M i
1 s'
r,/Pkc
'(Vf-^
•2TABLE OP CONTENTS
page
Abstract
..........................................................
3
I
Introduction .................................................
4
II
Experimental Considerations
9
A.
B.
C.
D.
E.
...............................
Introduction .............................................
9
Materials.................................................... 10
E q u ipment.................................................... 11
Operating Procedures ....................................
13
Analytical Procedures...................................... 16
III
Discussion of Methods of Data A n a l y s i s .................... 17
IV
Discussion of R e s u l t s ........................................... 22
A.
B.
C.
D.
Effect
Effect
Effect
Effect
of
of
of
of
Tenperature......................................
P r e s s u r e ......................................
Molecular Weight
...........................
Structure on Hydrogenation of Butyl Amine
.
24
25
2$
26
V
C o n c l u s i o n .................................................... 32
VI
A c k n o w l e d g e m e n t s ............................................... 33
VII
Literature Cited .............................................
34
VIII A p p e n d i x ........................................................ 35
145253
-3ABSTR AC T
• The effects of pressure, temperature, and space velocity on the
hydrodenitrogenation of certain aliphatic amines were studied.
The feed
concentration and hydrogen rate were held constant for all runs.
The.com­
pounds studied were n^butyl amine, octyl a m i n e , .iso-butyl ■amine., secbutyl--amine, a n d .tert-butyl amine.
i
An increase of temperature caused a corresponding increase in the
conversion of the nitrogen compound.
An increase in pressure also caused
an increase in the nitrogen conversion.
By comparing n-butyl amine to octyl amine,.it was evident that the
lower molecular weight amine denitrogenated more readily.
Similarly,..the
isomers of butyl amine denitrogenated in the order n-butyl amine s isobutyl amine, .■sec-butyl amine and tert-butyl amine., with the ease of deni.trogenation increasing respectively. ' The product formed from n-butyl
amine is a mixture of iso-butane and h -butane. Sec-butyl amine forms
essentially n - b u t a n e , while iso- and tert-butyl amine form essentially
iso-butane at high conversions.
-4—
I.INTRODUCTION.
When .a nation's industrial picture is changing rapidly* new.proc­
esses and products.must.he.introduced at a corresponding rate.
But
with each new or improved p r o c e s s ^ more demands are placed •on each inter.?mediateosiepp:-.-uThesedeman'ds may be quite, diversified,, but .one of the
largest demands falls on the- specifications.and requirements of an inter­
mediate used.
This rapidly.changing industrial picture applies directly
to-most chemical, and petroleum industries..
With any.industrial changes or.new growths ..new.markets are created.
To -supply, these .markets larger.and different sources or raw materials
and intermediates are needed.
Often new sources -of these materials
must be used,, which may. .require the .use. of inferior grades.
This imme­
diately, presents the problem-of upgrading the materials to meet the
required specifications.
Demands to. improve any product present;'new
problems' that must.be- met .and h a n d l e d ,
If the information needed-to '
obtain a ,complete knowledge.of the problem is not available.,.then it
may.be necessary.to- conduct studies to obtain this information.
In recent years, one problem has arisen that exemplifies this
demand.
This problem.concerns, itself with the efficient removal of
nitrogen-containing compounds from the petroleum.distillates■of which
t h e y sarepa .part.
Since 1953 the Esso Research and Engineering Company has sponsored
a research project at Montana State College.
Not only was this study
I
-5conducted here.* ,but also- In their, own research laboratories.
The
purpose of the.project was to study.the removal of sulfur by hydro­
desulfurization which also included in-cycle catalyst.deactivation
studies
(6 , ,g.jt-ll, l 6) .
Recently,, however, ..the study has been
carried to the level where it is no.longer desirable to continue .
this..type of,work -at the present time.
Since 195$ and.the .com­
pletion of Mahughts thesis., attention has been focused on hydrode<-.
nitrogenation.
Because of •previous experience, with desulfurization.,
and the similarities between this and denitrogenation* this new
..study, was conducted in much the same way. as desulfurization.
In an effort, to understand .better a.small portion of the.:over-■
■all .problemj,,. this study, was conducted on Qnly,one type of nitrogen
-compound..
The type of nitrogen compound .used.in this study was
limited.,to/ aliphatic.amines... h o w e v e r ,. this- type o f .compound makes
up a.part -of the total nitrogen content of' many commercial..petro^
•Ieum feedstocks . ■T h e 'e£E'gx$ts up on hydrogenation, of stiCh..variables
as pressure.,., temperature*..space-velocity J,. and-structure, of the.
amines, were studied^ and their correlations to hydrogenation were
■examined.
The feedstock used in this study- was blended.to -resemble -some
o f .the ,characteristics of a commercial, feed.
However3..only, one
type, of nitrogen compound of known concentration was introduced
into.the feed.
This-.made it.possible to understand better the
-6behavior of this compound to hydrogenation.
Commercial feeds may contain several types of nitrogen coim­
pounds .. Some of the more common compounds encountered are as
follows:
I.
H
R - C H
NH 2
Amlnes
Quinoline
Isoqulnollne
Pyridine
Pyrrole
H
6 . Figurations undetermined
Porphyrins
A feedstock may contain many more types of nitrogen com­
pounds than these.
Also, each type of compound may have more than
one possible arrangement of atoms In its structure.
As an example,
quinoline differs from isoquinoline In only the placement of the
nitrogen atom in the heterocyclic ring.
Since many compounds are
present in most feedstocks, numerous reactions may occur simul­
taneously.
as f o l l o w s :
Some of the problems encountered in hydrogenation are
-71-
Does the ^pfesenoe of different nitrogen compounds cause
an interaction ttia-t would affect the hydrogenation?
.2,
Does the presence ,of c m p o p n d s other than nitrogen-con­
taining ,compounds affect hydrogenation?
3..
What affect- does the- carrier h a y e on. hydrogenation?
-4.
What part of the reaction is rate controlling?
5-.
What,physical conditions arid limits'
-6 .
How do variables like pressure and. temperature affect the
ape present?
hydrogenation?
In most cases before a study like this is. conducted, there,
must first-he sufficient interest to merit such an investigation.
Reasons leading up to the.desire to remove nitrogen compounds are
numerous and diversified,,
-gome of the more.obvious reasons are as
follows:
1.,
When, nitrogen-containing compounds c o m e into -contact with,
sensitive catalysts^ the catalysts are easily poisoned
and quickly lose their efficiency.
Some processes in -
which this may occur are catalytic cracking., hydrogen
.platforming,, and alkylation (I,.
' 2..
10).,
Nitrogen compounds are- partially responsible for extensive
gum formation.,
TMs- formation is Caused, by their ability
to accelerate the oxidation of numerous unsaturated
compounds
(1,
5.)..
3.
Removal .of nitrogen compounds to produce .a product that
is competitive with those from other sources.
The re­
moval of nitrogen compounds from, shale ail to upgrade its
.quality would be, an example,
The catalytic hydrogenation
of crude shale ail has been studied by the Chemical
Engineering Department at Montana State College since
1954
.4.
(2 , 5 j- 7) v
Nitrogen compounds are.often the source,of objectionable
odors
(15)..
5 . Nitrogen impurities Can hinder various chemical processes
The purpose and. reasons f o r this st.udy have been previously
stated.
The information obtained f r o m this study is of funda­
mental nature and should be of value in solving the broader
p r o b l e m of upgrading -commercial feedstocks,
-9
II EXPERIMENTAL .CONSIDERATIONS
A.
Introduction
C a r r i er :
In order to- p r e p a re a feedstock that contained the
desired, amount of nitrogen as amines> it was necessary to use a
hydrocarbon carrier that simulated commercial feedstock charac^
teristics.
It was necessary to choose a carrier that in barrel
lots was reasonably"' p r i ced* .that would blend readily with various
low molecular weight amines* and not affect the hydrogenation
process itself.
The hydrocarbon carrier .chosen was normal h e p t a n e », which has
a boiling point of
98„5°C a n d a density of 0,6838 gm/cc (4),
The
•normal heptane was purchased in barrel lots from the Phillips
Petroleum Company,
Process Conditions:
The operating conditions chosen for this
study were orginally selected, with the assistance of the Esso
Research and Engineering Company.
After a few- exploratory runs
were conducted* the -conditions were modified, to prevent total
nitrogen conversion and. also to be more compatible with the limi­
tations of the equipment involved, and the analytical techniques
used.
The following .conditions .were finally selected:
Pressure — 10.0
$00 psig.
Temperature - 62$°?
725°]?.
Hydrogen flow rate -■ $00 SCE/bbl.
Nitrogen concentration - 1,6$ N by wt.
Space velocity - 10 — 100 gm/gm p er hour.
-tIOCatalyst -• CoMo-; 1 0 - 100 grains „
Amines .used: n-batyl amine
tent-butyl amine
sec-butyl amine
iso—butyl amine
n-octyl amine
B.
Materials
Feedstock:
The various amines u s e d in this study were com­
mercial grade reagents purchased f r o m Eastman Organic Chemicals.
The desired amine was blended with n-heptane to produce approxi­
mat e l y lfo by weight nitrogen,..
space velocities
Since each, run consisted, of four
(IO jl- 2 0 4 0 „. 100) * it w a s .nodessary to Jblend about
15 liters' of feed p e r run..
This quantity of feed was sufficient to
r u n the unit until the .nitrogen conversion reached a constant level
and samples obtained.
Catalyst a n d .catalyst s u p p ort:
Hydrodesulfurization w°rk con­
ducted previously at Montana. State College in. conjunction with, the
Esso Research and Engineering .Company j[-9>. 11) Used a cobaltmolybdenum catalyst..
Erom previous experience of hydfodenitro-
genation and from the similar characteristics between, desulfuri­
zation .and denitrOgenatIonj the Halco-Esso cobalt-molybdenum 1/16inch extruded catalyst was used..
This catalyst is a mixture of
cobalt and molybdenum, oxides on an al u m i n a .support.
T h e catalyst b e d was supported in the reactor on the top and
bottom by
1/ 8 -inch alundum pellets which were obtained from the
Morton Company.
The alundum pellets were also used to dilute the
-ll-.
catalyst and. thereby produce a larger catalyst zone.
The catalyst
was diluted j? parts pellets to I part catalyst by yolpme.
The cata­
lyst dilution made it possible to use a larger portion of the r e ­
ac t o r which aided .in. the control of reactor conditions.
Hydrogen Treat G a s The hydrogen treat gas was supplied in.
h i g h press u r e .cylinders by H R Oxygen .and Supply.,. Billingsjc Montana.
The h y d r o g e n was first passed through a "Peoxp" to remove trace
quantities of oxygen,.
A palladium, catalyst in the 'tBeoxo" unit
catalytic ally combined the oxygen with hydrogen to form water.,
The
water was then removed b y passing the hydrogen through a drying
,unit which was p a c k e d with "Drierite".
C»
Equipment
Elow D i a g r a m :
A schematic flow .diagram of the catalytic
hydrogenation unit is shown, in Figure I.
The feed is pumped to
the top of the reactor where it enters along .with the purified
hydrogen.
Together the f e e d and hydrogen pass down through the
preheater, catalyst^ and after-heat zone.
The vapors are.con­
densed first in a counter current water condenser while still
under reactor pressure..
After passing through the reactor pres­
sure regulator^ the gas a n d liquid products ar e then passed
through a cooling .coil .contained in a n ice b a t h .
The liquid p r o ­
duct is collected in .a flask while the gaseous product is vented
to the atmosphere.
-12EqiiilBment Specifications :
seamless,^ schedule
length.
The reactor was made frqm I -Inch O D j
8 O j stainless steel .pipe,f;and .was 30 Inches In
The bottom -of the reactor was silver welded to a flanged
union to permit easy access to the Inside,of the reactor.
The top
,of the reactor was permanently, connected to a .high pressure cross.
A thermowell enters the reactor through the top of the cr o s s . •The
feed, and hydrogen enter the r e a c t o r .through one side of the., cross
while the.other is used as a. safety device to vent the reactor to
the atmosphere.
A 1 5 0 0 psi rupture disk was used for this purpose.
The r e a c t o r wras wrapped-with five, ceramic-beaded nichrome
heating co i l s .
The heating coils were covered with approximately
1-1/2 inches .of .magnesia, insulation.
A thermowell extended .down through the center of the reactor,
to the .flanged, ,union at the bottom.
A 3/l6^inch OD stainless
steel.tube-y sealed at the lower end,^ .was u s e d for the thermowell.,
.Inside the thermowell were placed fiye lron^constantau thermo­
couples which measured the temperature at various leyels through
the reactor.
A diagram of the location -of heating coils.* thermo­
couples^. •and catalyst aone are shown in Figure.2.
Accessory equipment was also utilized i n the hydrogenation
unit as f o l lows: .A HIlls-McCanna high pressure proportioning
.pump with a l/ 8-inch piston;, a Brooks armored, high pressure roto.meter with 3/52-inch b a l l 5 a drove back pressure regulator) five
-13110 volt Powers tats ;j a 100Q ml glass feed reseryoir with a.
50 ml
graduated burette attached through a side arm* a Leeds and
Worthrup. indicating potentlffimdter,* four Marshalltown. 200.0 psi test
g a u g e s ; a Qohor Deoxp P u r ifier 5.a Matheson hydrogen regulator.
'
The tubing used,on the,unit was type 304- stainless steely 1 /8-,inch.
OD tubing.
Various', types of yalyes used on the .unit are -as follows:
Hoke- on-off yalv.e; Hoke turn-to-open needle .yalye j .Hoke micro,
adjusting needle valve used for hydrogen metering..
D.-
Operating Procedures
Reactor Preparation.:
Once the reactor is. dismantled from the
•system it i s .cleaneduand dried before recharging.
The reactor is
inverted to expose the annular space between the reactor wall and
the thermowell.
The alundum. pellets were first charged into the
reactor to a selected depth,.of the reactor.
This forms the- feed preheat section
The,catalyst that had been diluted with pellets
was then, charged, into the reactor and, p a c k e d Jpy tapping.
The
length of the .catalyst ,zone was kept as .uniform ,as possible for
each run.
The reactor was then filled to .about one inch below
the flange with more .catalyst support...
A .stainless steel coil
Vfas then secured.on the- bottom, of the reactor to hold .the catalyst
and catalyst support in place when it was turned up into its n o r ­
m a l position.
The reactor was then coupled into its position in
-14the system..
The feed and gas line> the thermocouple leads,,, and the Power­
'stat cords were then connected, to the reactor.
Qnce the reactor,
was pressurized,, the unit >ras checked for l e a k s ,
After the reactor was brought to a temperature of 288"0, a
gas mixture of
5$ hydrogen sulfide and. 95$ hydrogen was passed,
over the.catalyst for a period of 24 hours at a reactor pressure
of 250 psig.
This procedure was used for catalyst hydrodenitro-
genation run s t a r t s .
Reactor Operation.:- Before the actual run was started, the
reactor was changed from, the break-in temperature to the desired
temperature fo,r that particular run.
Once the pump was started
it was necessary to adjust the pumping ,rate to the ..desired, rate.
This was done by a micro,-adjustor on the pump that changed the
stroke length of the piston.
The space velocity was set to the
desired .value by .measuring the volumetric oil feed, r a t e .
As the
f e e d and hydrogen entered the reactor it was necessary to adjust
the preheat Powerstat to heat the incoming feed to reactor tem­
perature.
The temperatures through the reactor were recorded,
and the Powerstats were continually adjusted to maintain the
desired constant temperaturet
The product passed out of the
reactor and the liquid w a s collected in a flask until a product
sample was taken.
-.15gampllng,:
Before a .prodtiet sample was taken,,, it was necessary
to wait for the reactor to line pat .and. thereby produce a. reasonably
•constant c.onversiqn.
quinoline that from,
Ryffel
(13). fo.und In his. investigation of
6 to 8 hours of line-out time was sufficient to
insure a constant conversion,
After sufficient line out time,, a
sample’ of about 50 cc was taken in a 500 ml flask.
This sample
was followed by another sample at approximately I hour l a f e r .
In
most cases both samples were analyzed and. the average of the two
conversions was used.
Since- the various amines are. in vapor phase
while in the reactor, it was necessary to be sure that all of the
amines that had not Jpeen converted to ammonia were.condensed and
collected in the product s a m p l e .
To, insure this^ the liquid p r o ­
duct .and, the gas product were passed, through,,an additional con­
denser after passing through the Grove regulator.
This condenser
was a.stainless steel.coil that was maintained in a n ice b a t h .
At the beginning of the study^ the additional condenser was
not used.
In Figure 3> two runs of identical conditions are
plotted^ except during ,one of the runs, the additional.condenser
was employed..
,It pan be seen that the. ice-bath h a d considerable -
effect on fully recovering the unreacted amines from, the gas
product stream.
Additional procedures were employed in, .an
effort to .determine if there was a n appreciable loss of amines
from the product due to carry oyer but this could, not b e fully
—1.6determlned.
E.
Analytical Procedures
The nitrogen, content of each product sample was determined ,by
the standard Kjeldahl method (8).
For each run it was necessary to
determine the nitrogen content of the feed, as well as the nitrogen
content of the various samples.
Each determination was run in
duplicate to insure accuracy,, and the average of the two trials was
used.
Samples f r o m the runs which studied, the effect of hydrogena­
tion of the isomers of n-butyl amine were further analyzed with a
vaporphase chromatography u n i t .
The use of this unit ,made it
possible to examine the .various intermediates and products formed
upon hydrogenation.
This work was accomplished with a n Aerograph
Model A-IlO-C gas chromatograph in conjunction with a BrownHoneyweil .Model 14,5 x.
57 I H V
recorder.
—3-7’
III DISCUSSION OP METHODS OP DATA ANALYSIS
The rate of a chemical reaction, is expressed, quantitatively
as the mass or ,moles of a product produced, or reactant consumed^
per unit time
(14).
The rate of reaction can also be termed the
velocity or speed of a specific reaction.
Since the mass or
moles of a product or reactant are usually expressed as a concen­
tration,; the rate of reaction is.dependent upon the change of
concentration with time.
If the rate is based on the .change of concentration (C) of
the reactant with time
(t)> the rate of reaction (r) can be exp.
pressed as:
since the concentration of the reactant is decreasing.
"The law of mass action .states that the rate of a chemi
cal .reaction is proportional to the product of the 'active
masses' of the reactants involved. Since the activity of
a substance in a mixture is frequently difficult to obtain,
concentrations are usually used to replace the active mass
terms.
Por example, in the reaction
A + B —> R + S
we can express the rate as
r = --
s kC% 8%
where r is the rate, C a and C^ are the Concentration of the
respective reactants A and B and a, b, and k are constants.
The constant k in the above equation is termed the 'specific
reaction rate c o n s t a n t ', or simply the 'rate.constant'.
In
, rrgineral, its .units depend upon those employed for the con-
—18 —
centrallon arid upon the order .of reaction.
The order of
reaction Is defined as the sxtoi of the' exponents a and. h.
Reactions orders m a y h a v e values of 0, I* 2^
or some
fractional value.. This derivation of the rate equation
based.upon the law of mass action is theoretically■valid
only for homogeneous systems. However* it has been found
that data f r o m heterogeneous systems can also, be correlated,
quite -well in many cases.
This is particularly true when
one of the reactants.is present in large excess,
In. a heterogeneous catalytic reaction^ a factor must
also be included in the .rate equation to account for the
preparation^ composition^, and. physical properties of the
catalyst.
Thus for the overall reaction of
Quinoline + Hydrogen — $=■Hydrocarbons + Ammonia
the rate could, be expressed a s ; s
1=--aF=kM cS.
where k is the rate constant> z is the catalyst factor* and
C a n d C are the concentrations of quinoline and hydrogen*
respectively.
This is* of course* assuming constant tem­
perature a n d pressure,." (13)
The above equation for this particular study would be replaced
by the reaction:
Amines + Hydrogen -— > Hydrocarbons + Ammonia
■
If a large concentration of hydrogen is used*, the change in con­
centration.of hydrogen is negligible-and therefore the concentration
is assumed to be constant.
For any one given catalyst^ the factor
z is a constant and .can be combined with the rate .constant
f o r m a new constant R 2 .
to
Since the product from the reaction is
analyzed for nitrogen,* and, the nitrogen concentration of the feed
is also, knovjn^ it is advisable to base -the change of concentration
-+19'.
in the reaction on the concentration of the nltfogen rather than
the particular nitrogen compound.
This changes.the above rate
equation to the .new form:
Since the amount of nitrogen remaining in the sample, is
analyzed the-diff erence between the. initial .concentration (A) a n d
the amount converted (x), is determined.
then be substituted, for
This quality. (A~x) can
In, equation (I,)..
Also^ the symbol (.'O) is
u s e d to represent time by common convention.,.
r = -^
Equation (I) becomes:
= .Ka(A-Tx).11
(2 )
Since the actual .contact time is virtually impossible to.
determine^ some proportional measure of contact time must b e used.
The term ,space velocity is used and it is based. Upon, the yolume or
weight of reaching mass per unit v o l u m e .or weight of catalyst p e r
unit time.
Since ,both the feed and catalyst are measured, in, the
same units.,, space velocity has the unit of reciprocal time.
By
using the reciprocal of space velocity? this quantity can be subr
Stitufced
for the actual contact time in the above e q u a t i o n F o r
this study, the space velocity was determined by the weight of feed
in ,grams to the yreight of the catalyst in grams per hour.
If equation (2) is rearranged and integrated from. x. = .0 to
x = x
and Q = 0 to & = Q, the following is truer
(3)
-2.0Where O is also replaced by l / S y
The order of ,the reaction, is then equal to the'exponent
equation, (3) -
(n) in
In. arriving at this- equation^ it, was assumed the
hy d r o g e n concentration was a constant,; therefore^, ,the order .of, the
reaction is termed, "pseudo order".
T o r experimental .methods of determining order of reaction^ the
different orders are usually assumed,until one is found, which agrees
with the experimental -data. , T h e •agreement between the order of
'
reaction tested and. the data is. ,usually tested, visually unt i l the
.data looks as though it fits quite well.
If additional assurance
is needed.* statistical methods can. b e u s e d to further verify the
exactness of the res,ttlts,
.Under any condition^ however* the d a t a
points should n o t s h o w ..a .definite Upward o r .downward trend j (3')
-In. this, study the following, orders were -assumed -and tested,;
n = I.* 3/2.* 2.
f o r the .case of n = I
P
O
. ,fe
.A-X
=
'
-%A.
Sy
which integrates to give ln( V a -x :). = K e/S^,
A plot, of I n ( V A 7X). ys
l/Sy would, then, yield, a straight line with a .slope .of ,Kg. and an'
intercept of zero,.
To study reaction, orders of 3/2 a n d .2* the f o l l owing.should
be plotted and the results examined to determine if the .data yields
a. .straight Ihie.;
n = 3/2
Plot
2 / (A-X-J1^ 2VS l / S y
Intercept u 2 / A ^
— 21—
n = 2
Plot
I / (A-x)
vs
Intercept - l/A
1/SV
This.discussion..is. IarSeiI, on the assumption that the reaction isAmines + Hyirogen —
Hydrocarbons •+ Ammonia.
Pnom the ,analysis
obtained Ir pm. the. chromatograph., it was .evident that no .intermedi­
ates tha/k .contained nitrogen were lprmed.,
.I t .is-.,reasonable to
assume that the nitrogen compounds were .entirely converted to
ammonia or else remained in the s a m p l e .as the original nitrogen
compound.
—22—
IY DISCUSSION. OF RESULTS
The data from -this study were first examined .in. the m a n n e r .out­
lined previously.
Two hydrogenation runs were selected for the
■study of possible, reaction rate's.
The two runs chosen for this
analysis were selected on the basis that they best represented.the
charaoteristies of all t h e .
■data,,,'and because these runs were con­
ducted with some of the operating conditions similar to each of the
other.runs.
The operating conditions along w i t h 'the initial feed concentra­
tion changed with each run.
Since this information is only used- in
analyzing the dafa^ it is not generally referred to in this report.
These data.are given in Table II.
Also, the data.contained in
Tables III,.IV, V,.and VI, are the data.used for.constructing
,Figures 4 through 10.
Figure 4
is a plot of In A / (Ar-X) vs reciprocal space Velocity.
Since the data do not even resemble a, straight line through the
origin for either.run.,, it is assumed t h e .reaction cafe between
n - b u t y l :amine and hydrogen 'under the giveh conditions is not first
order. .Similarly,.-Figures 5 and 6 are plots to determine if the
reaction is- second order, or 3/2. order, respectively.
In "Figure 5,,
l / (A-x) i s . p l o t t e d ,vs reciprocal space velocity with the- intercept
equdl to l/A.
In this figure, ..the run conducted at 2^0 psig
I
reactor pressure comes closer to .fitting.a straight l i n e , but is
StiXl not accupEtte enough to assume a second..order reaction.
Figure
6 indicates clearly that the reaction rate is not of the order 3/ 2 .
F r o m the ,basis of this analysis,, it is assumed that the.reaction
rate is not of -common o r d e r .
This does not •eliminate the possi­
bility of an empirical equation that could express the reaction rate
for hydrogenation of n— butyl -amine.
It is- possible that the reaction
is controlled by some particular-step which .must first occur before
the-actual hydrogenation reaction, is completed.
For example., if it
is first.necessary.to form a carbonium ion from the amine before
the reaction can occur., then this rearrangement would conceivably b e
the controlling.step in the reaction. -The .possibilities of -actually
forming carbonium.ions, in such a reaction are -discussed later.
Since .more -extensive -studies were, not conducted into possible reac­
tion c o n t r o l l i n g ^steps., an oyerall reaction rate expression cannot
be determined.
In an effort to compare the.results of these runs ^.a common
basis was chosen for the analysis of all the-data.
The method
chosen was t o 'h a n d l e .the data in a,manner-similar to that f o r a first
order.reaction.
This- method was chosen f o r two reasons.
Since this
work was carried on in conjunction with the Esso Research .and Engi­
neering Company.,.the data were analyzed in the form of a first order
reaction so that both groups used the -same- .method .of data analysis.
Secondly* it- has been shown that the rate- of reaction for the
Jaydrogenation of quinoline under somewhat similar operating -condi­
tions -was of first order (13 ).
A,
Effpet Pff temperature,:
The .effect of -temperature.-on
hydrodenitrogenation between the temperatures, of
shown in Fig u r e Th
625-to 7250B1 is
■Fop a ..plot of In ^/(A-x) ys reciprocal, space
Velocity,.. the.;Uurves. should ,have an intercept .of z e r o .
This condi­
tion is true -both from integrating the reaction rate equation and
froni the.fact that at an infinite space velocity,*-the conversion
would b e - z e r o ,
At h i g h space v e l o c i t i e s , .it became increasingly more, diffi­
cult to.maintain -constant.temperature.through the catalyst zone. .
At a. space velocity, of 100.* .the -upper part of the ..catalyst zone
was not maintained at the -desired temperature.
This happened
because it became impossible for the-preheat zone to supply suffi­
cient' heat to bring.such.a.large volume.of feed.up to reactor
temperature.
F o r .this, reason the .data obtained at a space velocity
of 100 were.not included in this .temperature stu d y ^
The -data for
space velocities of 40.or less were obtained at constant tempera­
ture conditions.
■From Figure tJf it is,evident that an increase in temperature
of 5Q°F through- the-reactor w a s .sufficient to increase the conver­
sion considerably.
As -an example., the -data obtained at a space
velocity of .20.can be.used to compare the amount of nitrogen
-25■removed, for each temperature.
At ..this, space, velocity the..-catalyst
zone could h e maintained-at constant temperature, .but yet the .-con.Version was not high en °ugh to create-possible lnacccuracies in the
nitrogen content determination.
At this.space velocity.the ,percent
of nitrogen r e m o v e d .ah .the, temperatures of
respectively-were 66., S 75*2^,. and @0.0#.
•over, this temperature, range -an increase of
625, 675., and 7? 5 0F
This, indicates.that
50°F.-created- a.corres­
ponding increase -of approximately 6, 6^ .in the total, a m o u n t .of
nitrogen removed.
B.
Effect of p r e s s u r e;
The.effect of pressure, on hydrode-
.nitrogenation f or. n-butyl amine .between the .pressures of IOQ and
500 psig is. shown .in'Figure 8..
Figure 8 is a.plot,of In. A/(A-x)
vs.reciprocal s p a c e .veloc i t y .
As the ,pressure increased., ,the conversion increased rapidly.
For a pressure .of 100 psig., a. s p a r e .velocity, of 10 gave a .conver­
sion of -approximately
65^.
At a. pressure of ^OO psig.., .the-space
velocity, was increased to 100 and still gave a conversion higher
than at a space, velocity, of 10 for a reactor pressure of 100 psig..
T h e .conversion at a..pressure, of 5PP psig.and a space velocity, of
100 was 65.8$,, while -a space velocity of 100 at 100 psig had -a
conversion.of 5 5 -.SfL
Three runs were compared i n ‘Figure 8-.
with a n-butyl.amine feed.
All runs were ,made
The,pressures were 100,■250,. and 500
^-26—
psig.
Again ,a. space velocity, cxf 20 was -chosen for a cpmpapison
between conversions,
The conversions f or pressures of 100^-250,
and 500 p s Ig ,were $1.?#, 80.0#, and 95-.2^ respectively.
space velocity of
At a
10 ,and-a pressure.of 500 pslg».there was essen­
tially. complete.conversion.
C.
Effe c t ,of molecular weight:
,As a- comparison for molecu­
lar weight both n-butyl smine a n d .n -octyl amine were studied.
N o r m a l .octyl amine was hydrogenated at pressures of
p s i g ,and a temperature of
plotted In Figure 9-
100 and 500
7250F « The results of these runs are
Also^ in Figure
•amine are .plotted for a comparison.
9 ,similar runs with n-butyl
At pressures of both 500 and
IOO psigj the conversion is hig h er f o r .n-butyl amine than for
.H-OCtyl amine.
toward'
As the .molecular.weight, increases ^ this trpnd
more stable- compounds would probably continue for. other
saturated aliphatic amines
The product from the n-octyl amine study was examined for
ppssible intermediate, compounds.
By-use of the .chromatograph it
was possible to determine that the n-octyl amine was not cracked
to form lower .molecular weight hydrocarbons .
dent that the •carrier, -n-heptane* underwent
Also.,.it- was evi­
very, little .cracking
or change of composition.
D.
Figure
. Effect of structure on .the .hydrogenation of butyl a m i n e s ;
10 shows the effect of hydrogenation on the,various isomers
-27of butyl a m i n e . .F r o m the Figure It is evident that the ease of
denltrogenating the Isomers decreases from tert-butyl amine.* secbutyl amine* Iso-butyl amine.., to ,n-butyl amine In that order.
a ■comparison between, the conversions , ..a^space velocity of
As
100 for
tert-butyl. amine gave much higher conversions than did a space
velocity of
10 for n-butyl amine.
In an effort to justify this occurrence*, various possible reac­
tion mechanisms were studied.
The possible intermediates were
examined by use of a vapor-phase chromatograph.
It was proposed
that the reaction occurred after the amine first went through a
rearrangement to form a. carbonium-ion.,
A carbonium ion. is a.group
of carbon atoms such that the carbonium. ion carbon has only.six
electrons i n its valence shell (12).
As an example of a carbonium
i o n * ■isOtbutyl amine can. f o r m the ion:
.H3C
i.
CH 3 - 0+
i
H 3C
■This ion .can then react with a hydrogen ion from the catalyst to
form the hydrocarbon isobutane.
The identification of the reaction products and intermediates
was accomplished, by using the method., of yapor-phase chromatography.
A vapor-phase chromatograph works essentially as a highly efficient
distillation column., ..However., it allows the analysis of very small
samples in a.matter of minutes.
The sample under investigation is
.-28in je eted into the iinit and is carried.,by a stream of helium into the
packed column where it is selectively absorbed on .the substrate..
■The absorption properties of each compound determine their
respective retention times in the column.
The stream of gas
emerging, f r p m the column passes through a sensing .element which
measures its thermal..conductivity and compares it to a.standard
(helium) .
This change i n thermal conductivity is shown -on .a
continuous chart recorder-.as a series, of peaks ? one for each com­
po u n d .as it emerges f r o m the column.
.Thus by identifying each
peak by. comparing it to peaks created f p o m k n o w n compounds ,■ it is
possible to determine the various intermediates.or products formed
in a reaction (13).
Samples studied by this method were:
1.
.n-butyl ,amine .under high conversion.
2 . iS'Q'-butyl amine under h i g h conversion.
3 . sec - b u t y l .amine under high conversion.
4. • t e r t - b u t y l .amine .under ,high conyers i o n .
3 . .Urrbutyl pmine under -low. conversion.
6.. iso-butyl-amine ..under low. conversion.
7 . sec- b u t y l -Umine Under l o w .conversion..
8 . tert-butyl-amine under low.conversion.
9 . iso-butyl amine with acid.wash.
10.. .tert-butyl-amine with acid wash.
The chromatograms of the above studies are included in Figures
11 and 12.
In these figures* the Various products formed a r e also
identified.
The proposed mechanism for hydrodenitrogenation, of the isomerp
of butyl-amine is essentially that of a car.bonium ion rearrangement.
-29Both n-butyl amine-and iso-butyl amine form the carbonium ion before,
reacting.• The .sec-butyl amine, reacts directly without rearrange­
ment while tert-butyl .amine reacts as a carbonium ion since it is
already in that, form ('12).
Samples f r o m these.runs which had,very high conversions seemed
to uphold this theory.
This assumption is based,on the fact that
the carbonium. ion. must first be.formed before.the reaction can
occur.
Because of this.,,, -some .of the hydrocarbons.produced must
s'
appear in. the product as isobutane.
■E i g u r e .11 shows the. results
of this investigation.
The.difference between the isobutane curve ,and.the n-butane
curve is labeled (A).on Figure.11.
By comparing the s e .c u r v e s •to
the .curves-' produced ,from the product, samples.,.it is. possible to
•distinguish .the :various hydrocarbons -which were formed.
Part
qf Figure 11.shows.the effect of the n-butyl.amine s a mple.
(B)
By
comparison .it is.evident that some isobutane is formed from n-butyl
amine.
Also,, Part
(O) shows that iso-butyl,amine produces essen­
tially iso butane at high .conversions,
Part
(D) indicates that sec-
butyl -amihe-does not ppodp.ee,-any. Isobutane.!,therefore.,,the. carbonium
ion was.probably n o t .formed,before denitrogenation occurred. Finally,
Part
(E) indicates that, tert-butyl.amine,produced isobutane .-upon
■reaction...
This .analysis at high conversions tends to.uphold.the
carbonium ion rearrangement as .a, possible intermediate .step.,in the
-90reaction.
As .-a further check,.■samples from the .same.studies...at low. con^
versions.w e r e .examined.
Part
(A) of Figure 12 indicates that at
l o w e r .conversions.,.n -butyl amine, still, produces, some isohutane .and
Part '(B) indicates, that sec-hutyl-amine still forms, only, n-hufane.
However, iso-butyl .amine.and., tert-hufyl amine indicate that, there
is. some, other intermediate formed.in addition to. the isobutane..
This is sh otm..as Part
('C).and (B) in. Figurp 12.
The .possibility
that these two .compounds form, an intermediate.of n - b u t a p e .is diffi­
c u l t .t o :understand .or explain,.
.In a n e f f o r t .to--determine the.
nature of this intermediate, compound,,, the .samples, were acid-washedbefore b eing .examined..-on-the chromatograph.
acid wash are Part
(E) and
The. s.amples. after­
(F) of Figure 12.* iso-butyl-amine and
tert - b u t y l .amin^.-respectively.
However,., f.rpm-the .Figure.it is
evident that the-acid .-wash did. not remove the intermediates . -If
■ these intermediates had been unsaturated.or-perhaps some a m i n e ,
the -acid-wash sho u l d .h a y e .r e m o v ed,t h e s e .compounds .
Since.the
carrier has been shown to ..not he affected.by. the hydrogenation, ,it
is difficult t.p -determine fully the nature of these intermediates.
The true.mechanism for.these -reactions cannot be fully determined
,.withp.ut.further inves tigation,
The .feed-samples for each-compound were-also examined for
impurities^ but since no impurities.were found, this analysis is
not reported- in the ,appendix.
The.possibility that,.these compounds must, form various inter­
mediates before ..reacting, may be part .-of the-reason'.that. the. reac­
tion rate cou,ld not be.expressed as.some.simple.order.
i "
By assuming such ,a mechanism .for t h e .hydrogenation, it would
then ,be' possible .to explain- ttie-order,in '.which these, compounds'
^enitrogenated,
Using the carbonium ion..mechanism.as an example.,,
the f o l l o w i n g .mny.occur.
If the tert-butyl amine-did not -rearrange
-and.was. alre a d y .in ,-a. form which -caused,-direct .reaction.,,.then .it
would yield.the highest conversions.
If the secondary compound
also did not' BeaMaiager ^hpt -was not in the-some active, state-as
tert-butyl.amine,, then It would ,yield,high.-conversions.but not as
high ..as.tert-butyl a m i n e .
The .iso-butyl amine -would -undergo some,rearrangement^, but
.not as much,as .would -nsbutyl amine.
•This would ,cause.the iso-
butyl amine.to-react faster than the n-b-ptyl.amine.
This, t h e n / .
means that-the-amines.reacted.in-the,order with this.rearrangement
mechanism.
•From. Figure.10, it can be seen Lthat these.compounds
.did -.denitrogenate in this, particular, order.
V ''CONCLUSIONS
The data; from the .hydrqdenitrogenatlon atudy were f i r s t .-examined
for possible pseudo reaction rate.order. .It was determined that
the reaction rate was not of common order.
From the .chromatograph
analysis,:it was evident that n-butyl amine .formed'intermediate
compounds upon hydrogenation.
The formation of intermediates.could
.be.rate controlling, causing the .order .of reaction.rate to be .of
uncommon order.
The effect o f .temperature wfcs.studied on .n-butyl amine.
It
was shown that an increase in temperature increased the total
nitrogen conversion.
At a space .velocity of.20, an increase in
temperature of ^O 0F .increased.the .total conversion by
6+$.
An increase in pressure increased, the total nitrogen conver­
sion considerably.
was
At a space velocity of 100, the conversion
65.8^ at 500 psig.as compared.to a.conversion,of 35.8$ -at 100
Pflg By comparing-.n-butyl amine to n-octyl amine, it was.evident
that the lower molecular- weight compound reacted.to give higher
conversions.
This condition,held fo r pressures.of 100 and 5QQ psig.
The conversions for the isomers of butyl amine decreased in
the order, of tertiary, secondary, iso, and. normal.butyl.amine.
This-order.may fee -accounted.for, by the.various-mechanisms needed
for each compound to denitrogenate.
VI ACKNOWLEDGEMENT
The author would.like.to take this opportunity to. acknow­
ledge and ..thank the Esso -Research, and Engineering .Company that
sponsored this research project and supplied many materials used
throughout the investigation.
He also wishes, to thank the staff
of the-Chemical Engineering .Department for their helpful suggest
tions and criticisms-;.and in particular. Doctor Lloyd. Befg who
directed this research.
He would also like to thunk.Mr.
G. P.
Schreiber^,fellow research worker,,..for his assistance .on this
project.
. V m B I T E R A T U B E .CITED
(1)
Ball,, J. S. ,., et a l -'"Nitrogen Content of Crude -Petroleum11)
I n d .■and E n g r .■C h e m .,.4^:25 (1951)•
(2)
Benson, D. B., B h .D .■ThesIs * Montana State College, (1958).
(5)
•Corrigan, T . E .,■"Chemical E ngineering,Fundamentals", C h e m .
F n g r vj.61 (-1.954),, 62(1955).
(4)
Hodgemun^l C. D.> and H. N- Holmes.,.-"Hand Book ..of Chemistry
and Physicsn j .Chemical -Rubber Publishing , C o Cleveland,
-Ohio (1955).
(5)
Holecek., -R. J.., M.S. Thesis., ,Montana State College,; (.1955) •
46)
Jacobson, R. L.., -Ph.D. Thesis.,. Montana State Collegef- (I958-) .
(7 )
King, G. A.., M.S. -Thesis;,. Montana State College,. (1955) •
(8 )
Kirkj .P . L. ,• "Kjeldahl .Method for Total Nitrogen"., Analytical
Ch e m . , 22:554(1950).
(9)
Mahugh, R. A. , Ph.D, Thesis^ Montana State . . C o l l e g e (1,960).
(10)
Mills, G. A., et a l . "Chemical,Characteristics of Catalysts.
I. Poisoning.of Cracking,Catalysts by Nitrogen Compounds.and
Potassium Ion." .J. Am. C h e m . Soc ., 7? ^ 5 4 4 (1950) .
(11)
Opprecht, M. K., M.S. Thesis,, Montana State College*
(12)
Royals E . E ., ■"Advanced .Organic Chemistry" , Pr entice-Hall,.
Inc.j New York., (1954) .
(15)
Ryffel,. J. R . f .Ph.D. Thesis* Montana State College,,
.(1.4)
Smithy J. M., "Chemical Engineering Kinetics'!,, McGraw-Hill
Book Co. , New ,York* (1956).
(15 )
United States Bureau,,of Mines,*,Report.of Investigation*,
(1958.) .
(i960).
5044(1951).
(1'6)
Waterman*.-R,. .
vG.,j, Ph.D. Thesis.* Montana State College*. (1958).
APPENDIX
page
Table I
Effect of Ice Bath on Sampling
35
Table .11
Operating .Conditions and Initial Feed
•Concentrations
36
Table III
Data for Test of Reaction Order
37
Table IV
D a t a for Effect of Temperature on Hydrogenation
.38
Table V
D a t a for Effect of Pressure on Hydrogenation
39
Table VI
Effect of Structure on Hydrogenation of Butyl. Amine 40
Figure I
Schematic Flow Diagram .of Hydrogenation Unit
41
Figure 2
Detailed Diagram of Reactor
42
Figure
3
Effect of fee Bath on Sampling
43
Figure
4
Test for
Pseudo First Order Reaction
44
Figure
5
Test for
Pseudo Second Order Reaction
4$
Figure
6
Test for
Pseudo Reaction Order of 3/2
46
Figure 7
'
Effect of Temperature on Hydrogenation
4y
Figure § :.
Effect of Pressure.on Hydrogenation
48
Figure 9
Effect of Pressure on Hydrogenation of
Aminej,.. and Effect of Molecular Weight ojL
Hydrogenation.
nvQctyl
49
Figure 10
Effect of Structure .o n .■Hydrogenation of Butyl Amine 50
Figure 11
Chrdmatogram for High Conversion Samples
Figure 12
Chromatogram for L q w ■Conversion Samples and. Ac,id
Washed. S a m p l e s ’
51
.52
-36table
.I
EFFECT OF- ICE ,BATH ON SAMPLING
Sample
Conversion
A-x
Space
Velocity
Ice
Bath
Sample
.Conversion
A-x
iclce
Space
V e l o c i t y • Bath
Sv
M
.
N- 2,^6
'
.06 0
-IQ
No.
.1262
,2 0
Ho
I
N-IO
'3 5 7
AO
.No-
I
'
,Yes
N —2^f
.2 0 2
20
Yes
N -2 A t 6
.3 8 7
' 40
Yes
■
Unifotim Operating ,Conditionst
Feed.:
Temperature:
.Pressure:.
.Weight.of Catalyst:
10
1% :n>-butyl amine
725'0F
250 psig
,10 g.
— 30’'TABLE II
.OPERATING CONDITIONS.AND.INITIAL FEED CONCENTRATIONS
.Run
Feed
Feed
Cone.
Wt..^N
T e m p .°F
Press.
Ps ig.
Space Velocities
Nr-14
n-butyl amine
0 .9 8 4
675
250
1 0 , 20
N^ l 6
;n-butyl amine
0 .9 7 ;
625
250
N-17
octyl amine
1 .0 5 0
725
500
.-10.,
Nr 18'
n-butyl amine
.1 .0 1 4
725
500
, 1 0 , 2.0, 4 0 ,,1 0 0
N-19
sec-butyl amine
1 .0 2 7
725
290
1 0 , 2 0 , . 4 0 , 100
N-20
iso-butyl amine
1 .0 2 2
725
250
.10., 2 0 , 4 0 ,-1 0 0
NC21
tert-butyl amine
.990
725
250
. 1 0 , - 2 0 , 4 0 , 100
725
100
1 0 , 2 0 , 4 0 ,.1 0 0
100
10.,. 2 0 , 4 0 , 100
■10,20
20j .4 0 , 100
N—22
octyl amine
1 .0 4 0
N-25
n-butyl amine.
.1 .0 4 5
N-24
nObutyl amine
1 .0 0 8
725
250
1 0 , 2 0 , 40
1 .0 0 8
625
250
40
N-24
n-butyl amine
725.
-
Uniform Operating.Conditions:
.Pressure:
.W e i g h t ■of Catalyst:
,Hydrogen -Rate:
250 psj.g
,10 g.
500 SCE of Ha Zbbl .of Feed
''/
TABLE .III
DATA FOR ,TEST OF REACTION ORDER
Sample
Space Velocity
.Sv
l/Sv
Press..
Psig
A-x
A
A-x
In
A.
A-x
N— 23-2
20
.05
100
..505
2.07
:728
N-23-4
10
.10
100
,365
2 .:87
1.054
N —.25— 6
40
.025
100
.605
1.73
N-23-8
100
.01
100
,671
’ 1.56
...443.
N-24-2
•10
,10
250
,108
9.58
2.23 ■
N-24-4
20
.05.
250
,202
4.99
1.61
N— 24-— 6
-40
.025,
2^0
,387
2.61
.96
.548
i
Sample
I
.A-x.
2
.A
.957
'1,955
A-x
1■.- - .
2 ..8l4
2,
I
A
A ,
1:045
N—23-2
1 .98.
N-23-4
- 2.74o
-3..31
N-23-6
1,65
2.57
N^23-"8
1,49
.-2,44
N-24-2
9.27
6.10
.996
N-24-4
4.44
ll_
■It
ri
4,95
N-24-6
3.22
II
n
it
2.59
11
It
11
H
It
it
M
II
11
.Uniform .Operating ,Conditions'
Feed.:
Temperature:.,
.Weight of Catalyst:
.Hydrogen Rate:
lfo .n^buf^l
72§°F.
.10 g.
500 SCF of
amine
H a Z b b l of feed
1.99
■_
.1.008
-3<9—
TABLE .IV
-
.
DATA FOR EFFECT OF TEMPERATURE .ON HYDROGENATION
Sample
Space Velocity
y /v
Tempv0F
A-x
A
A-x
'
In
A.
A-x
N-24-2
.10
725
.108
9,24
N-24-4
.20
725n
.202
4 .9 9
1.61
6
40
725
.387
2.61
.96
Nr-14-4
20
675
.265
3.71
1.31
N-14-6
10
675
,14-2
6.93
1.93
N - 1 6—2
20
625
.325
3,01
.1.10
N -I6-4
.10
625
.222
4.4
N-24-8.
40
625.
.526
1.92
Uniform Operating.Conditions :■
.Feed:
.Pressure:•
Weight ofCatalyst:Hydrogen R a t e :
Vfo .n-Untyl .amine
250 psig
10 g.
500 SC^ of H 3Zbbl
.
2,23
1.48
.652
TABLE .V
DATA FOR•EFFECT OF PRESSURE .ON HYDROGENATION
Sample
Space
Velocity
v/v
N-23-2 •
N-23--4
N-23-6
N-23^8
N— 24-2
N-24-4
N-24-6
N-18-2
N— l8-4
N- lb- 6
N-18--8
N-22-2
N-22-4
N-22-6
N-22-8
N-17-2
N-17-4
N-I 7-6
N-I 7-8
.20
10
40
100
10
20
40
.20
10
40
100
20'
10
40
100
20
10.
40
100
Press.
Psig
100
.100
.100
100
250
250
,250
30-0
A-x
A
- -A-x
•505
22097
2.#7
1.73
.1,56
9.34
4 .99
2.61
14.7
.365
.605
.671
.108
,202
.387
.069
In
A
A-x
.728
1.054
.548
.443
2 .2 3
M
.11
.96
2.70 ’
' Uniform Operating .Conditions;
.Temperature:
.Weight of Catalyatc
.,Hydrogen R a t e :
n -butyl amine
It
It
' Il
.1 .6,1
500
.complete conversion
500
6.00
.169
1.79
500 ' .347
2,93
1.075
100
1.643
4497
,633
.100
.520
.6o4
2.00
1.00
-747
1.375
.519
100
.8Q2
1.296
.259
500
11.0
2.40
.0956
500
'conversion top high
500
3.80
.1,334
.277
500
..412
2 .55
.936
Feed:
Feed
-1% n-Pptyl -.amine
Vfo n-oetyl amine
7.25°F
IO g.
$00 SCF of Ha/bb.1 of Feed
"
.it
' I!
It
,
n-oetyl amine
11 "
It
It
It
11
M
TABLE .VI
.EFFECT .OF- S T R U C T U R E ,QN HYDROGENATION OF BUTYL AMINE .
Space
Velocity
Feed
It
N-24-6
,11
20
.202
4 .99
-1.61
40
2.61
.96
100
.0)88
2 5.5
3.34-
.20
.0739
13.85
2.63
n
40
.519
3.21
1,167
n
.100
.531
.1.927
.657
.100
,1480
6..94
N-r21--@
tert-butyl amine
N -20-2
iso-butyl, -.amine
N-20-6.
N-20~8
N - 19-8
2.23
9.34
sec-butyl ,amine
Uniform. Operating ,Conditions-:
..Temperature-.
Pressure:
1
.Weight Qf Catalyst:..Hydrqgen. Rahe-
725°F
2 5 0 -psjig
10 g.
56-O UQF of H 2Zhbl
H
N-24-4
Vio-
g
n-butyl amine
A
A-x
iA
A-x
is
N-24-2
In
A-x.
—j
Sample
1.94
-42-
Q K
W O
CO
Eh CO
O CO
Ph U
W Eh
H
FQ
Ph CO
CO Cq
DRIER
ROTOMETER INDICATING
DRIERITE
k
DEOXO UNIT
p w
o ,-q
HYDROGEN
Figure I.
Schematic Flow Diagram of Hydrogenation Unit
-45 -
Thermowell
"Rupture Disk
■Catalyst
Support
■Pipe Wall
■Catalyst Bed
Asbestos Tape
•Nichrome
Heating Coll
■Catalyst
Support
Insulation
"Flanged Union
O " ^ Denote Thermowell Location
Figure 2.
Stainless
Steel Screen
Detailed Diagram of Reactor
-4ty-
0 - U s e of Ice Bath
-Normal Water Condenser
W t . % N in sample
(A-x)
A
Sy Space VelocityFigure 3.
Effect of Ice Bath on Sampling
-4^-
2.5
Z
&
T e m p . 725°F
Feed I % n-butyl amine
O — 250 psig
2.0
Z
/
/
A — 100 psig
f
1 .5
(A/A-x)
z
Z
1 .0
f5'V
Z
, 6°. >
Z
A
A
/
z
Z
.025
.05
.075
.10
l/Sv Reciprocal Space Velocity
Figure 4.
Test for Pseudo First Order Reaction
.125
-46-
O —
250 pslg
— 100 pslg
.025
.05
.075
.10
l/Sv Reciprocal Space Velocity
Figure 5.
Test for Pseudo Second Order Reaction
.125
-4fj-
O
-250 psig
— 100 pslg
.025
.05
.075
.10
V s y Reciprocal Space Velocity
Figure 6.
Test for Pseudo Reaction Order of 3/2
.125
□ — Temp.
O ~Temp.
-Temp.
(A/A-x )
A
l/Sv Reciprocal Space Velocity
Figure ?>.
Effect of Temperature on Hydrogenation
( V a -X)
-4^-
500 psig
100 psig
1/SV Reciprocal Space Velocity
Figure 8.
Effect of Pressure on Hydrogenation
0 — n-butyl Amine
n-octyl Amine
1/SV Reciprocal Space Velocity
Figure 9•
Effect of Pressure on Hydrogenation
of n-octyl A m i n e , and Effect of
Molecular Weight on Hydrogenation
(V A - x )
-$& -
O — n-butyl Amine
A -Iso-butyl Amine
Q -sec-butyl Amine
V - tert-butyl Amine
l/Sy Reciprocal Space Velocity
Figure 10.
Effect of Structure on Hydrogenation
of Butyl Amine
(T)
A iV
<V7> <1
i
(D
(^)
ISe b u T ^ n €.
H
(D
/I Tn -m o -y» I o-
®
I SobuTaI) t
4-
» - bu f a n e
f <•<J
H*
7>-bvTyl
fltn c n <
"sample
©
IS o - bo T y / a Tm,T i«
9
©
Sec - bu7y /
Qmme
§
©
T rtl
6mm e
H
Cf
0
13
*-b
O
4
M
H*
f
O
0
2
5
3
H-
§
M
1H
0>
W
» -
bvT^T* C
- bv T y I
S am p le
S am p le
S tm p le
I
Figure 12.
Chromatogram for Low Conversion Samples and Acid
Washed S amples.
U.1
■'S*
r!
" r
V »
©
^ir
4-
(^ -
>7- bvTy I
(g)-
S e c- Io c ty /
d-miTv# S+Tr*p\e
Am rne Tm*.
/So -
!,aty)
tfy n iT ’ -'
S a m p le
a-jni-pr Sa-mfle
r+>T- bvTy I
^7 771 Iit •#
TerT ' b u ty l
Qyrt noe
ia Tnpl-*
Sample
( actd
uoesbej)
MONTANA STATE UNIVERSITY LIBRARIES
3 1762 1001 3372 5
N i /8
C936c
cop.2
145253
Currie, R . A .
145253