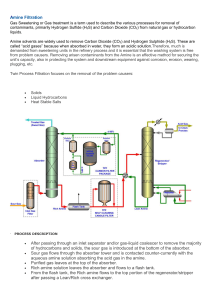
CH420/421 Practice Problems for Recitation on April 13, 2015
advertisement

CH420/421 Practice Problems for Recitation on April 13, 2015 1) Organocuprates are “soft” nucleophiles that undergo 1,4‐addition to ‐unsaturated ketones. Which of the following is the 1,4‐addition (conjugate addition) product? 2) Complete the following reaction schemes. O MeO O + CN NaOMe MeOH (aqueous workup) C8H11NO3 3) The 1,3‐diketone at right can be prepared by two different Claisen condensation reactions – one that forms bond a and one that forms bond b. Show the starting materials for each reaction. O O bond a bond b a. Precursor(s) for formation of bond a b. Precursor(s) for formation of bond b 4) What organic starting materials are needed to synthesize each compound using an aldol condensation or similar reaction? 5) Abilify® (aripipazole) is used to treat depression, schizophrenia, and bipolar disorder. Rank the nitrogen atoms in aripiprazole in order of basicity (1 = most basic, 3 = least basic). 6) In the reaction of (2‐bromoethyl)benzene with ammonia to synthesize phenethylamine: a. What is the main limitation of this procedure for preparing phenethylamine? b. How can the reaction conditions be adjusted to maximize the yield of phenethylamine? 7) An alternative approach to converting (2‐bromoethyl)benzene to phenethylamine is outlined below. Specify the appropriate reagents. 8) Treatment of amines with excess methyl iodide in the presence of silver oxide results in Hoffman elimination. In this reaction, the amine is alkylated to the quaterinary ammonium ion. Silver oxide exchanges iodide for hydroxide ion resulting in an E2 elimination reaction. Contrary to most other E2 elimination reactions, the least hindered proton is removed to generate the alkene product. Complete the following. 9) Reductive amination is a useful method of synthesizing amines. In this reaction, a primary or secondary amine is condensed with a ketone or aldehyde to generate an iminium ion intermediate. Subsequent reduction by NaBH(OAc)3 (sodium triacetoxyborohydride) provides the alkylated amine. Show this product. 10) Consider the formation of the secondary amine shown via reductive amination using agent NaBH(OAc)3 (NOTE: alternatively, NaBH3CN can be used as the reducing agent). There are TWO DIFFERENT pairs of starting materials, each if which is made of 9 or fewer carbons, that can be used to form the same amine product. Draw in the two possible combinations of starting materials. 11) What is the relationship between the following two structures? a. Resonance forms N b. Enantiomers N H c. Diastereomers d. Tautomers HN N