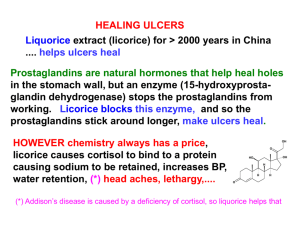
Claire Shoemake
advertisement

Claire Shoemake A DEFINITION y Structure-activity relationship (SAR) is the relationship between the chemical or three-dimensional structure of a molecule and its biological activity. y The analysis of SAR enables the determination of the chemical groups responsible for evoking a target biological effect in the organism. y This allows modification of the effect or the potency of a bioactive compound (typically a drug) by changing its chemical structure. y Medicinal chemists use the techniques of chemical synthesis & computational drug design to insert new chemical groups into the biomedical compound and test the modifications for their biological effects. y This method was refined to build mathematical relationships between the chemical structure and the biological activity, known as quantitative structure-activity relationships (QSAR). THE SAR PARADOX y The basic assumption for all molecule based hypotheses is that similar molecules have similar activities. y This principle is the basis of Structure-Activity Relationship (SAR). The underlying problem is therefore how to define a small difference on a molecular level, since each kind of activity, e.g. reaction ability, biotransformation ability, solubility, target activity, and so on, might depend on another difference. y In general, one is more interested in finding strong trends. y Created hypotheses usually rely on a finite number of chemical data. y The SAR paradox refers to the fact that it is not the case that all similar molecules have similar activities. HISTAMINE & ITS RECEPTOR SUBTYPES y The biogenic amine histamine plays an important role in a variety of pathophysiological conditions. y In peripheral tissues, histamine is mainly stored in mast cells and basophils. y In allergic conditions, histamine is released from these cells and is responsible for several of the well known symptoms of allergic conditions of the skin and airways. y In the gastric mucosa, gastrin induced histamine release fulfills an important physiological role by stimulating parietal cells to secrete gastric acid. HISTAMINE & ITS RECEPTOR SUBTYPES y In the CNS, histamine is synthesized in specific neurons that are localized in the tuberomammillary nucleus of the posterior hypothalamus. y These neurons project to all major brain areas and are involved in a variety of important physiological functions, including the regulation of the sleep-wake cycle, cardiovascular control, regulation of the hypothalamic pituitary adrenal-axis (HPA-axis), learning and memory. y Histamine exerts its action via at least four distinct receptor subtypes. Molecular biological approaches have shown that all y histamine receptors belong to the large family of G proteincoupled receptors. HISTAMINE & ITS RECEPTOR SUBTYPES y The gene encoding the H3 receptor has only recently been cloned. In contrast to the H1 and H2 receptor gene, the H3 receptor gene contains intronic sequences, leading to the identification of various H3 receptor isoforms following alternative splicing ofthe introns. y The isoforms show distinct expression patterns and signal transduction mechanisms. y Using the H3 receptor sequence, a new histamine (H4) receptor was identified ‘in silico’. y This receptor shows the strongest homology to the H3 receptor and also recognizes histamine with high affinity. HISTAMINE & ITS RECEPTOR SUBTYPES y For the H4 receptor, no pharmacological correlates are currently y known. y The H1 receptor couples mainly to Gq/11 thereby stimulating phospholipase C, y The H2 receptor interacts with Gs to activate adenylyl cyclase. y The histamine H3 and H4 receptors couple to Gi proteins to inhibit adenylyl cyclase, and to stimulate MAPK in the case of the H3 receptor. y In view of the important role of H1 and H2 receptors in allergic responses and gastric acid secretion respectively, many potent and selective antagonists have been developed as successful drugs. HISTAMINE & ITS RECEPTOR SUBTYPES y Selective agonists are currently also available as pharmacological tools. y The H3 receptor was originally described as an autoreceptor, inhibiting the release of histamine from histaminergic neurons in brain. y Recently, it was shown that this inhibitory effect is due to constitutive activity of the H3 receptor. y Recent evidence suggests that the H3 receptor regulates the release of several important neurotransmitters (e.g. acetylcholine, dopamine, GABA, norepinephrine, serotonin), both in the peripheral and central nervous systems. HISTAMINE & ITS RECEPTOR SUBTYPES y Highly potent and selective agonists and antagonists have recently been developed for the H3 receptor. These ligands are useful pharmacological tools and are currently being assessed for their clinical potential in allergy, inflammatory disorders, attention deficit hyperactivity disorder, Alzheimer’s disease and obesity. y The H4 receptor is highly expressed in peripheral blood leukocytes and intestinal tissue, making this receptor a potentially interesting target in allergic and inflammatory diseases. y The receptor shows high affinity for several H3 receptor ligands y (both agonists and antagonists), but shows a clearly different pharmacological profile. HISTAMINE & ITS RECEPTOR SUBTYPES y These data strongly suggest that the discovery of selective H4 histamine receptor ligands can be expected. y Because of the availability of many potent and subtype selective ligands for histamine receptor subtypes, good radioligands are y available. y For the H1 receptor, the antagonist [3H]-mepyramine has been successfully used in many preparations. The radioligand has nanomolar affinity and shows high specificity, although in liver y membranes, for example, binding to cytochrome P450 isoenzymes masks H1 receptor binding. y For in vivo Positron Emission Tomography (PET) studies, [13C]y doxepin can be used to label H1 receptors. This ligand has been used to label central H1 receptors in human brain. HISTAMINE & ITS RECEPTOR SUBTYPES y For the H2 receptor, the antagonist [125I]-iodoaminopotentidine y has recently been developed as a high affinity radioligand. y Because of its high sensitivity and subnanomolar affinity, this radioligand has been a very useful tool for characterizing y the H2 receptor. y As for the H1 receptor, an agonist radioligand is lacking. y In contrast, potent agonists and antagonists are y available for the H3 receptor, some in radiolabeled form. Initially, the agonists Nα-methylhistamine and (R)-α-methylhistamine y were developed as tritiated radioligands. HISTAMINE & ITS RECEPTOR SUBTYPES y Both ligands show selective, high affinity labeling of the H3 receptor with almost no non-specific binding. y The iodonated ligand [125I]-iodoproxyfan can also be used as an agonist radioligand. Originally described as an antagonist, iodoproxyfan acts as a partial agonist insome H3 receptor models. y [125I]-Iodophenpropit, [3H]-GR168320 and [3H]-clobenpropit can be used as H3 receptor antagonist radioligands. y For the H4 receptor, [3H]-histamine can be used to label the receptor protein. HISTAMINE & ITS RECEPTOR SUBTYPES HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y The term antihistamine historically has referred to drugs that antagonize the actions of histamine at H1-receptors rather than H2-receptors. y The development of antihistamine drugs began more than 5 decades ago with the discovery that piperoxan was able to protect animals from the bronchial spasm induced by histamine. y This finding was followed by the synthesis of a number of Nphenylethylenediamines with antihistaminic activities superior to piperoxan. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y Further traditional structure-activity studies in this series based largely on the principles of isosterism and functional group modification led to the introduction in the 1940s to 1970s of a variety of H1-antagonists containing the diarylalkylamine framework. y These H1-antagonists, referred to now as the firstmgeneration or classical antihistamines, are related structurally and include a number of aminoalkyl ethers, ethylenediamines, piperazines, propylamines, phenothiazines and dibenzocycloheptenes. y In addition to H1-receptor antagonists, these compounds display an array of other pharmacological activities which contribute toward therapeutic applications and adverse reactions. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y More recently, a number of second generation or “non-sedating” antihistamines have been developed and introduced. y The second generation agents bear some structural resemblance to the first generation agents, but have been modified to be more specific in action and limited in their distribution profiles. y H1-antagonists may be defined as those drugs that competitively inhibit the action of histamine on tissues containing H1receptors. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y The structural features required for effective interaction with these receptors will be discussed later on in this module. y It should be noted that some H1-antagonists also block histamine release. However the concentrations required to do so are considerably greater than those required to produce significant histamine receptor blockade. y The H1-antagonists do not block antibody production or antigenantibody interactions. y The H1-antagonists are now commonly subdivided into two broad groups - the first generation or classical antihistamines and the second generation or “non-sedating” antihistamines – based primarily on their general pharmacological profiles. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y The differences between these two series are discussed in more detail in the sections that follow. y It is important to note, however, that most detailed structure-activity analyses for H1-antagonists that have been published focus on the structural requirements for the first generation agents. y From these studies the basic structural requirements for H1-receptor antagonism have been identified as shown on the following slide: HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y Ar is aryl (including phenyl, substituted phenyl, and heteroaryl groups such as 2-pyridyl), y Ar' is a second aryl or arylmethyl group. y This diaryl substitution pattern is present in both the first and second generation antihistamines and is essential for significant H1-receptor affinity. y Furthermore several SAR studies suggest that the two aryl moieties must be capable of adopting a non coplanar conformation relative to each other for optimal interaction with the H1-receptor. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y The two aromatic systems may be linked as in the tricyclic antihistamines (phenothiazines, dibenzocycloheptanes and heptenes, etc.), but again they must be non-coplanar for effective receptor interaction. y Most H1-antagonists contain substituents in one of the aryl rings (usually benzene), and these influence antihistamine potency, as well as biodisposition as is discussed forindividual classes of compounds in the sections that follow. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y A basic, terminal amine function which in many of the first generation or classical antihistamines the terminal nitrogen atom is a simple dimethylamino moiety. y However, the amine may also be part of a heterocyclic structure, as illustrated by the piperazine, some propylamines (pyrrolidines y and piperdines), some phenothiazines, the dibenzocycloheptenes and the second generation antihistamines. y In all cases the amino moiety is basic with pKas ranging from 8.5 to 10 and thus presumed to be protonated when bound on the receptor. y The moiety is also important in the development of stable, solid dosage forms through salt formation. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y X is a connecting atom of O, C or N. The X connecting moiety of typical H1-antagonists may be a saturated carbon-oxygen moiety or simply a carbon or nitrogen atom. y This group, along with the carbon chain appear (see below) to serve primarily as a spacer group for the key pharmacophoric moieties. y Many of the anthistamines containing a carbon atom in the connecting moiety are chiral, and exhibit stereoselective receptor binding. y For example, in the pheniramine series and carbinoxamine, this atom is chiral and in vitro analyses indicate that those enantiomers with the S-configuration have higher H1-receptor affinity. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y The (CH2)n group represents a carbon chain which in typical H1antagonists consists of two or three atoms. y The (CH2)n group and connecting atom results in a distance between the central point of the diaryl ring system and the terminal nitrogen atom in the extended conformation of the antihistamines ranging from 5 to 6 angstroms (a "spacer" group). y A similar distance between these key moieties is observed for those antihistamines with less conformational freedom. y In some series branching of the carbon chain results in a reduction of antihistaminic activity. However, there are exceptions as evidence by promethazine which has a greater activity than its non branched counterpart. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y When the carbon adjacent to the terminal nitrogen atom is branched, the possibility of asymmetry exists. y However, stereoselective H1-receptor antagonism typically is not observed when chirality exists at this site. y Also, in those compounds which possess an asymmetrically substituted unsaturated carbon chain (pyrrobutamine and triprolidine), one geometric isomer typically displays higher receptor affinity than the other. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y Generally, the first and second generation anthistamines are substantially more lipophilic than the endogenous agonist histamine (or the H2-antagonists). y This lipophilicity difference results primarily from the presence of the two aryl rings, and the substituted amino moieties, and thus may simply reflect the different structural requirements for antagonist versus agonist action at H1-receptors. y The nature of the connecting moiety and the structural nature of the aryl moieties have been used to classify the anithistamines as indicated in the sections that follow. HISTAMINE H1 RECEPTOR ANTAGONISTS: ANTIHISTAMINES y Furthermore variations in the diaryl groups, X connecting moieties and the nature of substitution in the alkyl side chain or terminal nitrogen among the various drugs accounts for differences observed in antagonist potency as well as pharmacologic, biodisposition and adverse reaction profiles. y The ability of these drugs to display an array of pharmacologic activities is due largely to the fact that they contain the basic pharmacophore required for binding to muscarinic as well as adrenergic, serotonergic receptors. y The relationships of antihistamine structure to these overlapping actions (H1-antagonist, anticholinergic, and local anesthetic) are described later on. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y Antihistaminic Action: y The classical antihistamines have been used extensively for thesymptomatic treatment (sneezing, rhinorrhea, and itching of eyes, nose, and throat) of allergic rhinitis (hay fever, pollinosis), chronic idiopathic urticaria and a number of other histamine related,diseases. y These uses are clearly attributable to their antagonism of the action of histamine at peripheral H1 receptors. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y Although the symptoms of the common cold might be modified by antihistamines, these agents do not prevent or cure colds nor do they shorten the course of the disease. y The antihistamines also are of little or no value in diseases such as systemic anaphylaxis and bronchial asthma, in which autacoids other than histamine are important HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y Other Therapeutic Actions: y A number of the antihistamines, particularly the phenothiazines and aminoalkyl ethers, have antiemetic actions and thus may be useful in the treatment of nausea, vomiting and motion sickness. y Those agents which produce pronounced sedation have applications as nonprescription sleeping aids. y Several of the phenothiazines have limited utility in Parkinsonlike syndromes as a result of their ability to block central muscarinic receptors. y A number of antihistamines including promethazine, pyrilamine, tripelennamine and diphenhydramine display local anaesthetic activity that may be therapeutically useful. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y As the general pharmacologic profiles above suggest, the majority of antihistamines are capable of interaction with a variety of neurotransmitter receptors and other biomacromolecular targets. y This is most evident among the first generation agents many of which function as antagonists at muscarinic receptors and, to a lesser extent, adrenergic, serotonergic and dopamine receptors. y While some of these non-target receptor interactions may be of some therapeutic value (as discussed above), more frequently they are manifested as adverse reactions that limit drug use. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y This is particularly true of the peripheral anticholingeric effects produced by these drugs, and interactions with a number of neurotransmitter systems in the CNS that result in sedation, fatigue and dizziness. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y The primary objective of antihistamine research over the past 20 years has centered on development of new drugs with higher selectivity for H1-receptors and lacking undesirable CNS actions. y The pronounced sedative effects of some of the first generation agents were thought to result from the ability of these drugs to penetrate the blood-brain barrier, due to their lipophilic nature, and then block cerebral H1-receptors and possibly other receptors. y Thus research efforts were initiated to design novel antihistamines with reduced ability to penetrate the CNS and decreased affinity for central histamine receptors. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y These efforts led to the introduction the second generation antihistamines which are non-sedating and have little antagonist activity at other neurotransmitter receptors at therapeutic concentrations. y The pharmacologic properties of these agents are discussed in more detail later in this module. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y Surprisingly little information is available concerning the pharmacokinetic and biodisposition profiles of the first generation antihistamines. y Generally the compounds are orally active and well absorbed, but oral bioavailability may be limited by first pass metabolism. y The metabolites formed depend on drug structure to a large extent, but commonly involve the tertiary amino moiety. y This functionality may be subject to succesive oxidative Ndealkylation, deamination, and amino acid conjugation of the resultant acid. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y The amine group may also undergo N-oxidation, which may be reversible, or direct glucuronide conjugation. y Those first generation agents with unsubstituted and activated aromatic rings (phenothiazines) may undergo aromatic hydroxylation to yield phenols, which may be eliminated as conjugates. HISTAMINE H1 RECEPTOR ANTAGONISTS: Pharmacology y The H1-antagonists display a variety of significant drug interactions when co-administered with other therapeutic agents. y For example, monoamine oxidase inhibitors prolong and intensify the anticholinergic actions of the antihistamines. y Also, the sedative effects of these agents may potentiate the depressant activity of barbiturates, alcohol, narcotic analgesics and other depressants. y In recent years it has been discovered that several of the second generation antihistamines may produce life-threatening arrhythmias when co-administered with drugs that inhibit their metabolism. y These interactions are discussed in more detail in the sections that follow. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethanolamines) y The aminoalkyl ether antihistamines are characterized by the presence of a CHO connecting moiety (X) and a two or three carbon atom chain as the linking moiety between the key diaryl and tertiary amino groups. y Clemastine and diphenylpyraline differ from this basic structural pattern in that the basic nitrogen moiety and at least part of the carbon chain is part of a heterocyclic ring system, and that there are three carbon atoms between the oxygen and nitrogen atoms. 1st GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethanolamines) y The simple diphenyl derivative diphenhydramine was the first clinically useful member of the ethanolamine series and serves as the protoype. y In addition to antihistaminic action, diphenhydramine exhibits anticholinergic, antidyskinetic, antiemetic, antitussive, and sedative properties. y Diphenhydramine is not a highly active H1-antagonist. y Conversion to a quaternary ammonium salt does not alter the antihistaminic action greatly, but does increase the anticholinergic action. X = H: Diphenhydramine X = Br : Bromodiphenhydramine - Dimenhydrinate Carbinoxamine Maleate Doxylamine Succinate st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethanolamines) y Diphenylpyraline is structurally related to diphenhydramine with the aminoalkyl side chain incorporated in a piperidine ring. It is a potent antihistaminic, and the usual dose is 2 mg three or four times daily. y Clemastine Fumarate is structurally related to chlorodiphenhydramine with the aminoalkyl side chain incorporated in a pyrrolidine ring, and it has an additional benzylic methyl group. y This compound has two chiral centers, each of which is of the (R) absolute configuration in the dextrorotatory product. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethanolamines) y A comparison of the activities of the antipodes indicates that the asymmetric center close to the side chain nitrogen is of lesser importance to antihistaminic activity. y This member of the ethanolamine series is characterized by a long duration of action, with an activity that reaches a maximum in five to seven hours and persists for 10 to 12 hours. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethanolamines) y Drowsiness is a side effect common to the tertiary aminoalkyl ethers, presumably as a result of the ability of these compounds to penetrate and BBB and occupy central H1-receptors. y Although this side effect is exploited in over-the-counter (OTC) sleeping aids, it may interfere with the patient's performance of tasks requiring mental alertness. y The diaryl tertiary aminoalkyl ether structure that characterizes these compounds also serves as a pharmacophore for muscarinic receptors. y As a result the drugs in this group possess significant y anticholinergic activity, which may enhance the H1-blocking action on exocrine secretions. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethanolamines) y The frequency of gastrointestinal side effects in this series of antihistamines is relatively low compared to the ethylenediamine antihistamines covered later. y In spite of their extensive use, pharmacokinetic data on this series of compounds is relatively limited. y Most members of this series appear to be extensively metabolized by pathways including N-oxidation, and successive oxidative N-dealkylation followed by amino acid conjugation of the resultant acid metabolites st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethylenediamines) y The ethylenediamines were among the first useful antihistamines and are characterized by the presence of a nitrogen connecting atom (X) and a two carbon atom chain as the linking moiety between the key diaryl and tertiary amino moieties. y All compounds in this series are simple diarylethylenediamines except for antazoline in which the terminal amine and a portion of the carbon chain are included as part of an imidazoline ring system. y Because it differs significantly in its pharmacologic profile, antazoline is not always classified as an ethylenediamine derivative. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethylenediamines) y Phenbenzamine was the first clinically useful member of this class and served as the prototype for the development of more effective derivatives. y Replacement of the phenyl moiety of phenbenzamine with a 2pyridyl system yielded tripelennamine, a significantly more effective histamine receptor blocker. y Substitution of a para methoxy (pyrilamine or mepyramine), chloro (chloropyramine) or bromo (bromtripelennamine) results in a further enhancement in activity. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethylenediamines) y Replacement of the benzyl group of tripelennamine with a 2-thienylmethyl group provided methapyrilene, and replacement of tripelennamine’s 2-pyridyl group with a pyrimidinyl moiety (along with p-methoxy substitution) yielded thonzylamine, both which function as potent H1-receptor antagonists st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethylenediamines) y In all of these compounds the aliphatic or terminal amino group is significantly more basic than the nitrogen atom bonded to the diaryl moiety; the non-bonded electrons on the diaryl nitrogen are delocalized by the aromatic ring and the resultant reduction in electron density on nitrogen decreases basicity. y Thus the aliphatic amino group in the ethylenediamines is sufficiently basic for the formation of pharmaceutically useful salts. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethylenediamines) y The ethylenediamines also display a relatively high frequency of central nervous system depressant (sedation) and gastrointestinal side effects. y The anticholinergic and antiemetic actions of these compounds is relatively low compared to most other classical antihistamines. y The piperazine and phenothiazine-type antihistamines also contain the ethylenediamine moiety, but these agents are discussed separately because they exhibit significantly different pharmacologic properties. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Ethylenediamines) y Relatively little information is available concerning the pharmacokinetics of this series of compounds. y Tripelennamine is known to metabolized in man by Nglucuronidation, N-oxidation and pyridyl oxidation followed by phenol glucuronidation. y It is anticipated that other members of this series are similarly metabolized. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Piperazines/Cyclizines) y The piperazines or cyclizines can also be considered to be ethylenediamine derivatives or cyclic ethylenediamines (cyclizines), however in this series the connecting moiety (X) is a CHN group and the carbon chain, terminal amine functionality as well as the nitrogen atom of the connecting group are all part of a piperazine moiety. y Both nitrogen atoms in these compounds are aliphatic and thus display comparable basicities. y The primary structural differences within this series involves the nature of the para aromatic ring substituent (H or Cl) and, more importantly, the nature of the terminal piperazine nitrogen substituent. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Piperazines/Cyclizines) y Cyclizine and chlorcyclizine are simple Nmethylpiperazines. y Cyclizine HCl is used primarily in the prophylaxis and treatment of motion sickness. The lactate salt (Cyclizine Lactate Injection is used for intramuscular injection because of the limited water solubility of the hydrochloride. y Chlorcyclizine HCl has an additional ring Cl substituent which reduces activity. Chlorcyclizine is indicated in the symptomatic relief of urticaria, hay fever, and certain other allergic conditions. Cyclizine Chlorcyclizine st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Piperazines/Cyclizines) y Meclizine HCl and Buclizine HCl are N-benzyl substituted piperazines. Although it is a moderately potent antihistaminic, meclizine is used primarily as an antinauseant in the prevention and treatment of motion sickness and in the treatment of nausea and vomiting associated with vertigo and radiation sickness. y Buclizine Hydrochloride, is highly lipid-soluble and has central nervous system depressant, antiemetic, and antihistaminic properties. Meclizine Hydroxyzine Buclizine st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Piperazines/Cyclizines) y The piperazines are moderately potent antihistaminics with a lower incidence of drowsiness. y The activity of the piperazine-type antihistaminics is characterized by a slow onset and long duration of action. y These agents exhibit peripheral and central antimuscarinic activity and this may be responsible for the antiemetic (medullary chemoreceptor trigger zone) and antivertigo (diminish y vestibular stimulation) effects. y Thus as a group, these agents are probably more useful as antiemetics and antinauseants and in the treatment of motion sickness. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Piperazines/Cyclizines) y Some members of this series have exhibited a strong teratogenic potential, inducing a number of malformations in rats. y Norchlorcyclizine, a metabolite of these piperazines, was proposed to be responsible for the teratogenic effects of the parent drugs. y Metabolic studies in this series of compounds have focused primarily on cyclizine and chlorcyclizine, and these compounds undergo similar biotransformation. y The primary pathways involve N-oxidation and N-demethylation, and both of these metabolites are devoid of antihistaminic activity. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Propylamines) y The propylamine antihistamines are characterized structurally by an sp3 or sp2 carbon connecting atom with a carbon chain of two additional carbons linking the key tertiary amino and diaryl pharmacophore moieties. y Those propylamines with a saturated carbon connecting moiety are commonly referred to as the pheniramines. y All of the pheniramines consist of a phenyl and 2-pyridyl aryl groups, and a terminal dimethylamino moiety. y These compounds differ only in the phenyl substituent at the para-position; H (pheniramine), Cl (chlorpheniramine) and Br (brompheniramine). st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Propylamines) y The halogenated pheniramines are significantly more potent (2050 times) and have a longer duration of action than the parent pheniramine. y All of pheniramines are chiral molecules and are marketed as racemates or the individual active dextro-enantiomers as indicated below. y The halogen-substituted derivatives have been resolved by crystallization of salts formed with d-tartaric acid and antihistaminic activity resides almost exclusively in the Sstereoisomers y In addition to being an histamine H1 receptor antagonist, chlorphenamine has been shown to work as a serotoninnorepinephrine reuptake inhibitor or SNRI. y Brompheniramine led to the discovery of the SSRI zimelidine. Cl H 3 H CH3 N 3 N Br H Pheniramine H CH 3 N N H H CH Brompheniramine 3 H H CH3 Chlorpheniramine st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Propylamines) y Those propylamines with an unsaturated connecting moiety include the open derivatives pyrrobutamine and triprolidine, and the cyclic analogues dimethindene and phenindamine. y The conformational rigidity of the unsaturated propylamines has provided a useful model to determine distances between the key diaryl and tertiary pharmacophoric moieties in H1-receptor antagonists, a distance of 5-6.Å y For pyrrobutamine and triprolidine the E-geometric isomers are active. y The relative potency of triprolidine is of the same order as that of dexchlorpheniramine. Triprolidine Pyrrobutamine st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Propylamines) y The antihistamines of the propylamine group are among the most active H1-antagonists. y The agents of this class also produce less sedation than the other classical antihistamines (yet a significant proportion of patients do experience this effect), and have little antiemetic action. y They do, however, exhibit a signficant degree of anticholinergic activity, albeit less than the aminoalkyl ethers and phenothiazines. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Propylamines) y In the propylamine series the pharmacokinetics of chlorpheniramine have been studied most extensively in humans. y Oral bioavailability is relatively low (30-50%) and may be limited by first pass metabolism. y The primary metabolites for this compound, and other members of this series, are the mono- and di-N-dealkylation products. y Complete oxidation of the terminal amino moiety followed by glycine conjugation has also been reported for brompheniramine. y Chlorpheniramine plasma half-lives range from about 12 hours to 28 hours, depending on the route of administration (oral versus IV). st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Phenothiazines) y Beginning in the mid-1940s, several antihistaminic drugs have been discovered as a result of bridging the aryl units of agents related to the ethylenediamines. y The search for effective antimalarials led to the investigation of phenothiazine derivatives in which the bridging entity is sulfur. In subsequent testing, the phenothiazine class of drugs was discovered to have not only antihistaminic activity, but also a pharmacologic profile of its own, considerably different from that of the ethylenediamines. y Thus began the era of the useful psychotherapeutic agent. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Phenothiazines) y The phenothiazine derivatives that display therapeutically useful antihistaminic actions contain a two or three carbon atom, branched alkyl chain between the ring system and terminal nitrogen atom. y This differs significantly from the phenothiazine antipsychotic series in which an unbranched propyl chain is required. y The phenothiazines with a three carbon bridge between nitrogen atoms are more potent in vitro. y Also, unlike the phenothiazine antipsychotics, the heterocyclic ring of the antihistamines is unsubstituted. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Phenothiazines) y Promethazine, the parent member of this series, is moderately potent by present-day standards with prolonged action and pronounced sedative side effects. y In addition to its antihistaminic action, it is a potent antiemetic, anticholingeric and sedating agent, and significantly potentiates the action of analgesic and sedative drugs. y The other members of this series display a similar pharmacologic profile and thus may cause drowsiness and so may impair the ability to perform tasks requiring alertness. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Phenothiazines) y Also, concurrent administration of alcoholic beverages and other central nervous system depressants with the phenothiazines should be avoided. y In general, lengthening of the side chain and substitution of lipophilic groups in the 2-position of the aromatic ring results in compounds with decreased antihistaminic activity and increased psychotherapeutic properties st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Phenothiazines) y The enantiomers of promethazine have been resolved and have similar antihistaminic and other pharmacologic properties. y This is in contrast with studies of the pheniramines and carbinoxamine compounds in which the chiral center is closer to the aromatic feature of the molecule. y Asymmetry appears to be of less influence on antihistaminic activity when the chiral center lies near the positively charged side chain nitrogen. y While little pharmacokinetic data is available for the phenothiazine antihistamines, the metabolism of the close structural analogue promethazine has been studied in detail. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Aminoalkyl Ethers (Phenothiazines) y This compound undergoes mono and di-N-dealkylation, sulfur oxidation, aromatic oxidation at the 3-position to yield the phenol and N-oxidation. A number of these metabolites, particularly the phenol, may yield glucuronide conjugates. y It is expected that the phenothiazine antihistamines would display similar metabolic profiles. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Dibenzocycloheptenes/heptanes y The dibenzocycloheptene and heptane antihistamines may be regarded as phenothiazine analogues in which the sulfur atom has been replaced by an isosteric vinyl group (cyproheptadine) or a saturated ethyl bridge (azatadine), and the ring nitrogen replaced by an sp2 carbon atom. y The two members of this series are closely related in structure; azatadine is an aza (pyridyl) isostere of cyproheptadine in which the 10,11-double bond is reduced. y Cyproheptadine HCl possesses both an antihistamine and an antiserotonin activity and is used as an antipruritic agent. st 1 GENERATION H1‐ANTAGONIST DRUG CLASSES‐ Dibenzocycloheptenes/heptanes y Sedation is the most prominent side effect, and this is usually brief, disappearing after three or four days of treatment. y Azatadine maleate: A potent, long-acting antihistaminic with antiserotonin activity. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES y The second generation antihistamines are more similar pharmacologically than structurally. y Structurally they are all diaryl substituted piperazines (cetirizine) or piperidines (all others). y As discussed earlier in this module, these compounds were developed as selective H1-receptor antagonists with relatively high potency. y Most of these compounds also produce prolonged antihistaminic effects as a result of slow dissociation from H1-receptors, and the formation of active metabolites with similar receptor binding profiles. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES y The second generation agents have little affinity for muscarinic, adrenergic or serotonergic receptors and therefore display a lower degree of side effects associated with antagonism at these receptors, but their affinities for these receptors is somewhat variable. y Generally, the large aralkyl groups or polar groups linked to the piperidine/piperazine rings of these compounds reduces their affinity for muscarinic or adrenergic receptors. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES y Perhaps most importantly, all of these compounds are devoid of sedating effects at therapeutic concentrations due to poor CNS penetration, and possibly lowered affinities for central histaminic, cholinergic and adrenergic receptors. y While these compounds offer several advantages over the classical antihistamines, widespread use has revealed a number of therapeutic limitations. y This is probably most true for terfenadine and astemizole (since withdrawn) which have been found to produce life-threatening arrhythmias when used concurrently with drugs that inhibit their y metabolism. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES y These drug interactions have been most evident with the imidazole antifungals ketoconazole, itraconazole and fluconazole, and the macrolides erythromycin, clarithromycin and troleandomycin which inhibit the metabolism of terfenadine and astemizole, resulting in elevated levels of the parent drugs which are proarrhythmic. y This adverse effect is evident by prolongation of QTc intervals on ECG. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Terfenadine. Alpha-[4-(1,1-Dimethylethyl)phenyl] -4(hydroxydiphenylmethyl)-1-piperidinebutanol (Seldane®) is a reduced butyrophenone derivative of an aminoalcohol-type antihistaminic. y Terfenadine was developed during a search for new butyrophenone antipsychotic drugs as evident by the presence of the N-phenylbutanol substituent. It also contains a diphenylmethylpiperidine moiety analogous to that found in the piperazine antihistamines. y Terfenadine is a selective, longacting (>12 hours) H1-antagonist with little affinity for muscarinic, serotonergic or adrenergic receptors. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y The histamine receptor affinity of these compounds are believed to be related primarily to the presence of the diphenylmethylpiperidine moiety. y The prolonged action results from very slow dissociation from these receptors. y The lack of anticholinergic, adrenergic or serotonergic actions appears to be linked to the presence of the Nphenylbutanol substituent. y This substituent also limits distribution of terfenadine to the CNS. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Terfenadine is rapidly absorbed producing peak plasma levels in 1-2 hours. y The drug undergoes significant first pass metabolism with the predominant metabolite being fexofenadine, an active metabolite resulting from methyl group oxidation. y When drugs that inhibit this transformation, such as the imidazole antifungals and marolides, are used concurrently, terfenadine levels may rise to toxic levels, resulting in potentially fatal heart rhythm problems. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y This resulted in withdrawal of this drug product. y Terfenadine is highly plasma protein bound (97%) and has a half-life of about 20 hours. y Terfenadine is widely distributed in peripheral tissues, with highest concentrations in the liver. y The major route of elimination of terfenadine and its metabolites is in the faeces and elimination is biphasic. y The mean elimination half-life is 16-23 hours. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Fexofenadine is a selective peripheral H1-receptor blocker that, like terfenadine, produces no clinically significant anticholinergic effects or alpha1-adrenergic receptor blockade at therapeutic doses. y The lack of anticholinergic, adrenergic or serotoninergic actions appears to be linked to the presence of the N-phenylbutanol substituent which limits binding to these receptors. y No sedative or other CNS effects have been reported for this drug and animal studies indicate that fexofenadine does not cross the blood-brain barrier. y In vitro studies also suggest that, unlike terfenadine, fexofenadine does not block potassium channels in cardiocytes. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Furthermore in drug interaction studies, no prolongation of the QTc interval or related heart rhythm abnormalities were detected when administered concurrently with erythromycin or ketoconazole. y Fexofenadine is rapidly absorbed after oral administration producing peak serum concentrations in about 2.5 hours. y Fexofenadine is 60-70% plasma protein bound. y Unlike its parent drug, only 5% of the total dose of fexofenadine is metabolized. y The remainder is excreted in the bile and urine and the mean elimination half-life is about 14 hours. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Astemizole, USP. 1-(4-Fluorobenzyl)-2-((1-(4methoxyphenyl)-4piperidyl)amino)benzimidazole (Hismanal®). y Astemizole was developed from a series of diphenylbutylpiperidine antihistamines in an effort to extend the duration of action. y During development it was discovered that this compound produced little sedation or autonomic side effect. y Astemizole is a selective and long acting H1-antagonist with little affinity for muscarinic, serotoninergic, adrenergic receptors or H2-receptors. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Generally, both the diaryl system and large aralkyl group linked to the piperidine nitrogen appears to reduce its affinity for muscarinic or adrenergic receptors. y The piperidinoaminobenzimidazole moiety appears to be required for H1-receptor affinity, and contributes significantly to the persistent receptor binding that results in prolonged action. y potent and longer acting than terfenadine. y It does not penetrate the CNS readily, thus sedation and other CNS side effects (dizziness, drowsiness, fatigue) are minimal. y Astemizole also has no local anaesthetic actions. y It is used for seasonal allergic rhinitis and chronic urticaria. It has a slow onset of action (2 to 3 days). 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Astemizole is rapidly and completely absorbed orally and should be administered 1 hour before meals. y Peak plasma levels are observed within 1-4 hours. y Astemizole is widely distributed in peripheral tissues, with highest concentrations attained in the liver, pancreas and adrenal glands. y It undergoes extensive first pass metabolism by processes including aromatic hydroxylation, oxidative y dealkylation and glucuronidation. y The main metabolites are desmethylastemizole, 6-hydroxy y desmethylastemizole and norastemizole. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y The desmethyl metabolite has antihistaminic activity comparable to the parent drug, and thus contributes to the prolonged duration of action. y Astemizole is highly protein bound (96%) and has a plasma half-life of 1.6 days. y The apparent half-life of the desmethyl metabolite ranges from 10-20 days, depending on frequency of dosing of the parent drug. y The primary route of elimination is in the faeces. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Astemizole was discovered by Janssen in 1977 y It was withdrawn from the market because it produced life threatening arrhythmia in a minority of subjects y Recently there has been renewed interest in Astemizole owing to the fact that it was found to be a potent treatment for malaria. y It has a mechanism of action similar to chloroquine but has activity even in chloroquine-resistant parasites. Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ (2006). "A clinical drug library screen identifies astemizole as an antimalarial agent". Nat Chem Biol 2 (8): 415–16 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Loratadine, USP. 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene-1-carboxylic acid ethyl ester. y Loratadine is structurally related to the antihistamines azatadine and cyproheptadine. y It differs from azatadine in that a neutral carbamate group has replaced the basic tertiary amino moiety, and the phenyl ring has been substituted with a chlorine atom. y The replacement of the basic group with a neutral functionality is believed to preserve antihistaminic action while reducing CNS effects. y Loratadine is also structurally related to a number of tricyclic antidepressants. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Loratadine is a selective peripheral H1-antagonist with a receptor binding profile like the other members of this series, except that it has more antiserotoninergic activity. y Thus it produces no substantial CNS or autonomic side effects. y Loratadine displays potency comparable to astemizole y and greater than terfenadine. y Loratadine is rapidly absorbed after oral administration producing peak plasma levels in about 1.5 hours. y This drug is extensively metabolized, primarily to the descarboethoxy metabolite which retains some antihistaminic activity. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Both the parent drug and metabolite have elimination half-lives ranging from 8-15 hours. y The metabolite is excreted renally as a conjugate. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperazines y Cetirizine: [2-[4-[(4-chlorophenyl)phenylmethyl]-1piperazinyl]ethoxy]acetic acid (Zyrtec®). y This compound is a racemic compound. y Cetirizine is the primary acid metabolite of hydroxyzine resulting y from complete oxidation of the primary alcohol moiety. y This compound is zwitterionic and relatively polar and thus does not penetrate the blood-brain barrier readily. y Prior to its introduction in the US cetirizine was one of the most widely prescribed H1-antihistamines in Europe. y It is highly selective in its interaction with various hormonal binding sites and highly potent (» terfenadine) as y well. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperazines y The advantages of this compound appear to be once-daily dosing, a rapid onset of activity, minimized CNS effects and a lack of clinically significant effects on cardiac rhythm when administered with imidazole antifungals and macrolide antibiotics. y The onset of action is within 20 to 60 minutes in most patients. y Cetirizine produces qualitatively different effects on psychomotor/psychophysical functions compared to the first generation antihistamines. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperazines y However the most common adverse reaction associated with cetirizine is dose-related somnolence and thus patients should be advised that cetirizine may interfer with the performance of certain psychomotor/psychophysical activities. y Other effects of this drug include fatigue, dry mouth, pharyngitis and dizziness. y Because the drug is primarily eliminated by a renal route, its adverse reactions may be more pronounced in individuals suffering from renal insufficiency. y No cardiotoxic effects, such as QT prolongation, are observed with the new drug when used at its recommended or higher doses or when coadministered with imidazole antifungals and macrolide antibiotics. y However, other typical drug interactions of H1-antihistamines apply to cetirizine. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperazines y Dose proportional Cmax values are achieved within 1 hour of oral administration of cetirizine. y Food slows the rate of cetirizine absorption but does not affect the overall extent. y Consistent with the polar nature of this carboxylic acid drug, less than 10% of peak plasma levels have been measured in the brain. y Cetirizine is not extensively metabolized and »70% of a 10 mg oral dose is excreted in the urine (>80% as unchanged drug) and 10% recovered in the feces. y The drug is highly protein bound and has a terminal half-life of 8.3 hours. y The clearance of cetirizine is reduced in elderly subjects as well as in renally and hepatically impaired patients 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Pyrrolidines y Acrivastine, USP. (E,E)-isomer. It is an analogue of triprolidine containing a carboxyethenyl moiety at the 6-position of the pyridyl ring. y Acrivastine shows antihistaminic potency and duration of action comparable to triprolidine. y Unlike triprolidine, acrivastine does not display significant anticholinergic activity at therapeutic concentrations. y Also, the enhanced polarity of this compound resulting from carboxyethenyl substitution limits BBB penetration and thus this compound produces less sedation than triprolidine. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Pyrrolidines y Limited pharmacokinetic data is available for this compound. y Orally administered drug has a half life of about 1.7 hours and a total body clearance of 4.4 mL/min/Kg. y The mean peak plasma concentrations are reported to vary widely, and the drug appears to penetrate the CNS poorly. y The metabolic fate of acrivastine has not been reported. THIRD GENERATION ANTIHISTAMINES y Third-generation H1-antihistamines are the active enantiomer (levocetirizine) or metabolite (desloratadine & fexofenadine) derivatives of second-generation drugs intended to have increased efficacy with fewer adverse drug reactions. y Indeed, fexofenadine is associated with a decreased risk of cardiac arrhythmia compared to terfenadine. y Second generation antihistamines (terfenadine, astemizole, loratadine and cetirizine), which block peripheral H1 receptors without penetrating the blood-brain barrier, were developed and introduced from 1981 onwards to provide comparable therapeutic benefit without the CNS side-effects. THIRD GENERATION ANTIHISTAMINES y Although largely successful in this goal, terfenadine and astemizole were found to cause potentially serious arrhythmias when plasma concentrations became elevated subsequent to impaired metabolism. y It was established that the cardiac toxicity was mainly due to the parent drugs. THIRD GENERATION ANTIHISTAMINES y As active metabolites could account for most of the clinical benefit, the goal for the third generation of antihistamines became to develop therapeutically active metabolites that were devoid of cardiac toxicity. y The first of these drugs, fexofenadine (the active metabolite of terfenadine), was approved in July 1996, after an unusually rapid development programme. Its introduction set a new standard of safety that led the FDA to request the withdrawal of terfenadine in 1997 on the grounds that a safer version of an equivalent drug was now available. y Norastemizole and descarboethoxy loratadine, the metabolites of astemizole and loratadine, respectively, are also in clinical development. These offer comparable or superior clinical benefits 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Fexofenadine Hydrochloride. (+/-)-4-[1-hydroxy-4-[4(hydroxydiphenylmethyl)-1-piperinyl]y butyl-α,α-dimethylbenzeneacetic acid (Allegra®). y This compound is marketed as a racemate and exists as a zwitterion in aqueous media at physiological pH. y Fexofenadine is a primary metabolite of terfenadine. y It was developed based on studies that revealed when terfenadine’s hepatic conversion to the fexofenadine was blocked by other drugs or disease, levels of the parent drug (terfenadine) rise resulting in heart rhythm problems. 2nd GENERATION H1‐ANTAGONIST DRUG CLASSES‐ The Piperidines y Subsequent clinical trials demonstrated that fexofenadine was not only active and effective in allergic disorders, but less cardiotoxic than terfenadine. y This led to the approval of fexofenadine as an alternative to relieve the symptoms of seasonal allergies. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y IN THE PAST: y Success in drug design largely due to serendipity (natural sources) y Analogues of naturally occurring molecules synthesised to improve activity and/or reduce side effects y Variations on a trial and error basis y Wasteful with respect to time and effort involved THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y IN THE RECENT PAST (Last 20 Years): y Emphasis on rational drug design y Drugs designed to interact with a known biological system y The cimetidine story is an excellent example of this approach. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y THE REMARKABLE ASPECT OF THE CIMETIDINE STORY y At the onset of the project there were no lead compounds, y AND....... y It was not even known whether or not the necessary receptor protein existed. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y IN THE PAST: y Success in drug design largely due to serendipity (natural sources) y Analogues of naturally occurring molecules synthesised to improve activity and/or reduce side effects y Variations on a trial and error basis y Wasteful with respect to time and effort involved THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y ULCER THERAPY IN 1964 y Methods available few and unsatisfactory y Ulcers localised erosions in mucous membranes of stomach or duodenum y Presence of gastric acid causes problem/aggravates problem/delays recovery y Untreated ulcer causes severe pain; internal bleeding; mortality y 1926: actor Rudolph Valentino dies at age 31 of perforated ulcer THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y ULCER THERAPY IN 1964 y Conventional treatment neutralisation of gastric acid by administration of bases such as Sodium Bicarbonate and Calcium Carbonate y High doses required to achieve neutralisation; relief temporary; side effects great y Surgery (removal of part of stomach) sometimes resorted to. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y PHYSIOLOGY: y Gastric acid (HCl) released by parietal cells which are autonomically inervated y Autonomic stimulation results in acetylcholine secretion from the nerve endings adjacent to the parietal cells y Acetylcholine crosses gap between nerve endings and parietal cells y Parietal cells activated- release of gastric acid into stomach. y Triggers: sight, smell or thought of food implying that gastric acid is released even before food has entered the stomach THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y PHYSIOLOGY: y Nerve signals also stimulate the antral region of the stomach which contains hormone producing cells known as G cells. y G cells release a hormone (a peptide called Gastrin) y Gastrin is also released as food passes through the stomach y Gastrin moves into blood supply and travels to the parietal cells further stimulating the release of gastric acid THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y IMPLICATION FROM A RATIONAL DRUG DESIGN POINT OF VIEW: y Release of gastric acid should be inhibited by: y Antagonists of the acetylcholine receptor (anticholinergic drugs) y Gastrin Receptor Antagonists y This thought process demonstrates a fundamental tenet in rational drug design, namely the understanding of the biological processes involved in the condition being targeted, and the identification of receptors which require modulation THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y RECEPTORS WITH THE POTENTIAL FOR SUCCESSFUL MODULATION: y ANTICHOLINERGICS: y Would block the cholinergic receptor in the parietal cells and inhibit the release of gastric acid y BUT... y They would also inhibit acetylcholine receptors in other parts of the body and cause unwanted side effects THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y MORE RATIONAL DRUG DESIGN THOUGHT PROCESSES: y WHAT IS THE DISTRIBUTION OF THE TARGET RECEPTOR IN THE HUMAN ORGANISM? y Are the receptors present exclusively in the target locus or are they widespread? y If present exclusively at target unwanted side effect probability is low. y If widespread, do sub-types exist? And if subtypes do exist, is distribution at different loci subtype determined? y IN SHORT, MAY THE IDENTIFIED TARGET BE FEASABLY USED? (Ref. Statement in Red Font on previous slide) THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y RECEPTORS WITH THE POTENTIAL FOR SUCCESSFUL MODULATION: y IDENTIFICATION OF A DRUG CAPABLE OF BLOCKING THE HORMONE GASTRIN y In the rational drug design process therefore, all efforts were then concentrated on the complete understanding of the gastrin release process THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y MORE RATIONAL DRUG DESIGN THOUGHT PROCESSES: y WHAT ARE THE BIOLOGICAL PATHWAYS INVOLVED IN THE RELEASE OF THE MEDIATOR THAT MUST BE CHANGED? y IS THERE A COMPLETE UNDERSTANDING OF THESE PATHWAYS? y CONTRIBUTIONS BY BIOCHEMISTS AND PHARMACOLOGISTS ARE ESSENTIAL AT THIS STAGE OF THE RATIONAL DRUG DESIGN STUDY THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y RECEPTORS WITH THE POTENTIAL FOR SUCCESSFUL MODULATION: y Current (1962) knowledge also associated histamine with gastric acid release stimulation. y Could therefore an antihistamine be effective in the treatment of gastric acid? y Leap of faith in that although the association between histamine and gastric acid release was known, the exact dynamics of the in vivo association were not understood. y Furthermore, conventional antihistamines failed to inhibit gastric acid release. τ 2 3 2 α 2 3 2 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y A STUDY OF HISTAMINE: y Histamine is made up of an imidazole ring whic can exist in two tautomeric forms. y Attached to the imidazole ring is a two carbon chain with a terminal αamino group. y The pKa of this amino group is 9.80 which means that at a plasma pH of 7.4, the side chain of histamine is 99.6% ionised. y The pKa of the imidazole ring is 5.74, implying that at pH 7.4, the ring exists in the unionised form. τ α THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y A STUDY OF HISTAMINE: y Whenever cell damage occurs, histamine is released stimulating the dilatation and increased permeability of small blood vessels. y This allows defensive cells, eg white blood cells, to be released from the blood supply to an area of tissue damage to combat any potential infection. y Unfortunately, allergy and irritation also cause histamine release and are responsible for clinical conditions including hay fever, rash and asthma. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y THEORY: TWO HISTAMINE RECEPTORS? y From where did this histamine approach therefore arise? y Scenario: Conventional antihistamines fail to have an effect on gastric acid release. y BUT.... y They also failed to inhibit other actions of histamine eg failed to fully inhibit the dilatation of blood vessels induced by histamine. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y THEORY: TWO HISTAMINE RECEPTORS? y Perhaps 2 types of histamine receptor exist? y Could it be that histamine, the natural messenger, is capable of acting as an agonist equally effectively at both, and does not distinguish between the two subtypes. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y MORE RATIONAL DRUG DESIGN THOUGHT PROCESSES: y IN RATIONAL DRUG DESIGN, THE DETERMINATION OF WHETHER OR NOT TARGET RECEPTOR SUB-TYPES EXIST IS PARAMOUNT IMPORTANCE y IF RECEPTOR SUBTYPES ARE DETERMINED TO EXIST, AND THE SUBTLE DIFFERENCES BETWEEN THEM ARE DETERMINED, THEN HIGHLY SELECTIVE DRUG MOLECULES MAY BE DESIGNED, CAPABLE OF ELICITING THE DESIRED PHARMACOLOGICAL EFFECTS FOR A REDUCED SIDE EFFECT PROFILE. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y THEORY: TWO HISTAMINE RECEPTORS? y Perhaps 2 types of histamine receptor exist? y If 2 types of histamine receptor exist, then theoretically (as highlighted in principle on the previous slide), it should be possible to design antagonist molecules capable of distinguishing between the receptor subtypes. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y THEORY: TWO HISTAMINE RECEPTORS? y Perhaps 2 types of histamine receptor exist? y And if this theory was correct, then further extrapolation would continue to suggest that the conventional antihistamines known in the early 60s were already selective, in that they were capable of inhibiting histamine receptors involved in inflammation (arbitrarily called H1 Receptors), and were unable to inhibit the proposed histamine receptors involved in gastric acid secretion (the proposed H2 Receptors) THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y THEORY: TWO HISTAMINE RECEPTORS? y PROBLEM: y To date no known antagonist for the proposed H2 receptors y Until such a compound found, it could not be certain that the H2 receptors even existed y No receptor was available to study y The aim was to design a selective antagonist for this hypothetical receptor THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y SEARCHING FOR A LEAD y With a theory and no lead molecule, histamine itself was an obvious starting point, because if histamine was stimulating the release of gastric acid by binding to a hypothetical H2 receptor, then clearly, histamine was being recognised by the receptor. y The task then was to vary the structure of histamine in such a way that it would still be recognised by the receptor, but bind in such a way that it acted as an antagonist rather than an agonist. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Determine how histamine itself was binding to its receptors. y Structure-activity studies on histamine and histamine analogues revealed that the binding requirements for histamine to the H1- and the proposed H2- receptors were slightly different: THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y H1- RECEPTOR REQUIREMENTS: y The side-chain had to have a positively charged nitrogen atom with at least one attached proton. (Quaternary ammonium salts which lacked such a proton were extremely weak in activity) y There had to be a flexible side chain N between the above cation and the heteroaromatic ring. .. y The heteroaromatic ring did not have to be imidazole in nature, but it did have to have a nitrogen atom SAR FOR AGONIST AT H1 RECEPTOR with a lone pair or electrons orthoto the side chain. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FOR THE PROPOSED H2- RECEPTOR, SAR STUDIES WERE CARRIED OUT TO DETERMINE WHETHER HISTAMINE ANALOGUES COULD BRING ABOUT THE PHYSIOLOGICAL EFFECTS PROPOSED FOR THIS RECEPTOR ie STIMULATING GASTRIC ACID RELEASE. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y H2- RECEPTOR REQUIREMENTS: y Essential SAR requirements were the same as for the H1receptor except that the heteroaromatic ring had to contain an amidine unit (HN-CH-N:) .. SAR FOR AGONIST AT PROPOSED H2 RECEPTOR THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Having gained a knowledge of structure activity relationships for histamine, the task was now a molecule which would be recognised by the H2 Receptor, but which would not activate it. y More clearly, an agonist had to be converted into an antagonist y It was consequently necessary to alter the way in which the molecule was bound to the receptor. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y Pictorially one can imagine histamine fitting into its receptor site and inducing a change in shape which switches on, or activates the receptor. y An antagonist might be found by adding a functional group which would bind to another binding region on the receptor and prevent the conformational change required for activation. y Pg 557 fig 18.8 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y To begin with, a study of known agonists and antagonists in other fields of medicinal chemistry was carried out. y The structural differences between agonists and antagonists for a particular receptor were identified and then similar alterations were attempted on histamine. y For example, fusing an aromatic ring onto noradrenaline had been a successful tactic used in the design of antagonists for the noradrenaline receptor. y This same tactic was attempted unsuccessfully with histamine. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Another approach which had been used unsuccessfully in the development of anticholinergic agents was the addition of non-polar, hydrophobic substituents. y This approach was also attempted unsuccessfully with histamine by attaching various alkyl and arylalkyl groups to different locations on the histamine skeleton. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y This latter strategy, did however, yield one interesting result which was relevant to later studies. y It was shown that 4methylhistamine was a highly selective H2-agonist, showing far greater activity for the H2- than for the H1- receptor. y This fact obviously led researchers to question why such a small simple alteration should result in such a drastic change in selectivity .. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y The selectivity observed suggested that 4-methylhistamine, and by inference histamine, must adopt 2 conformationsone to fit the H1- and the other to fit the H2- receptor. y Since 4-methylhistamine was more active at the H2receptor, then the implication was that the conformation required for the H2- receptor was a stable one for 4methylhistamine, whereas the conformation required for the H1- receptor is unstable for 4-methylhistamine THE CIMETIDINE STORY‐ A Rational Approach to Drug Design .. Stable conformation of 4-methylhistamine Selective for H2-Receptor .. Unstable conformation of 4-methylhistamine. Selective for H1- Receptor THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Up to this point thus, research had concentrated on searching for an additional hydrophobic binding region on the receptor y Then the focus of research switched to determine the effect of replacing the terminal α-NH3+ group with a variety of different polar functional groups. y The reasoning was that different polar groups could bond to the same region on the receptor as the NH3+ group, but that the geometry of bonding might be altered sufficiently to produce an antagonist THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y It was from this study that the first crucial breakthrough was achieved with the discovery that Nα-guanylhistamine was acting very weakly as an antagonist (partial agonist). y This means that Nα-guanylhistamine activates the H2- Receptor, but not to the same extent as histamine. y As a result, the amount of gastric acid released is lower. y Most significantly, as long as Nα-guanylhistamine is bound to the receptor, it prevents histamine from binding and thus prevents complete receptor activation. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y Nα- guanylhistamine: HN N C H2 H2 C NH NH2 + HN N NH2 HN N C H2 H2 + C N NH2 H NH2 C H2 H2 C NH NH2 NH2+ + HN N C H2 H2 C N NH2 H NH2 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y QUESTIONS: y Which part or parts of the Nα-guanylhistamine skeleton were responsible for the observed effects? y Was the guanidine group itself acting as an antagonist? THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Various guanidine structures were synthesised which lacked the imidazole ring, but none had the desired antagonist activity. y This means that both the imidazole ring and the guanidine group were required. y The structures of Nα-guanylhistamine and histamine were then compared. y Both structures contain an imidazole ring and a positively charged group linked by a 2 carbon bridge. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y The guanidine group is basic and protonated at pH 7.4 so that the analogue has a positive charge similar to histamine. y However, the charge on the guanidine group can be spread around a planar arrangement of 3 nitrogens, and can potentially be further away from the imidazole ring. y This leads to the possibility that the analogue could be interacting with a binding region on the receptor which is out of reach of histamine. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design p.560 Figs 18.13 and 18.14 Fig 18.14 histamine capable of reaching only the agonist region Fig 18.13 the analogue with extended functionality is capable of reaching either region. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y It was postulated that 2 alternative binding regions might be available for the cationic group- an agonist region where binding leads to activation of the receptor, and an antagonist binding region where binding does not activate the receptor. y If most of the analogue molecules bind to the agonist region, and the remainder bind to the antagonist region, then this could explain the partial agonist activity. y Regardless of the mode of binding, histamine would be prevented from binding, and an antagonism would be observed due to the percentage of Nα-guanylhistamine bound to the antagonist region. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y DEVELOPING THE LEAD- A CHELATION BONDING THEORY: y Variations were attempted in order to evaluate if an analogue could be made which binds only to the antagonist region. y An isothiourea was synthesised. In this structure the nitrogen nearest to the imidazole ring was replaced with a sulfur atom. y The positive charge in this molecule was restricted to the terminal portion of the chain . y The scope was that this latter should interact more strongly with the proposed antagonist binding region if this is indeed further away. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Antagonist activity did increase, but the compound was still a partial agonist, showing that binding was still possible to the agonist region. y Two other analogues were synthesised, where one of the terminal amino acids in the guanidine group was replaced with either a methylthio group or a methyl group. y Both the resulting structures were partial agonists, but with poorer antagonist activity. X= SMe; Me THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y From these results, it was concluded that both terminal amino groups were required for binding to the antagonist binding region. y It was proposed that the charged guanidine group was interacting with a charged carboxylate residue on the receptor via 2 hydrogen bonds. y If either of these terminal amino groups were absent, then binding would be weaker, resulting in a lower level of antagonism. H H2 X C C H2 HN N H + O O N H H N Strong Interaction H H + N H2 NH C C H2 HN N X RECEPTOR O - X = NH, S RECEPTOR O Weak Interaction X = Me, SMe THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y The chain was then extended from a 2-carbon to a 3-carbon unit in order to see what would happen if the guanidine group was moved further away from the imidazole ring. y The antagonist activity increased for the guanidine structure, but, strangely enough decreased for the isothiourea structure. y It was therefore proposed that with a chain length of 2 carbon units, hydrogen bonding to the receptor involved the terminal NH2 groups, but with a chain length of 3 carbonunits, hydrogen bonding involved one terminal NH2 group along with the NH group within the chain. GUANIDINE & ISOTHIOUREA STRUCTURES GUANIDINE‐ Increased Antagonist Activity ISOTHIOUREA‐ Decreased Antagonist Activity 2 2 2 2 2 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Support for this theory was provided by the fact that replacing one of the terminal NH2 groups in the guanidine analogue with SMe or Me did not adversely affect the antagonist activity. (Ref Graphic Next Slide) y This was completely different from the results obtained when similar changes were carried out on the 2-carbon bridged compound. (Ref Graphic Two Slides Over) PROPOSED BINDING INTERACTIONS FOR ANALOGUES OF DIFFERENT CHAIN LENGTH C H N NH H 2 C X HN 2 + NH 2 2 O _ ‐ RECEPTOR O X = S, NH HN N C H 2 H 2 C C H 2 + X N NH H 2 O _‐ O RECEPTOR X = NH2 SMe Me EFFECT OF VARYING THE GUANIDINE GROUP ON BINDING TO THE ANTAGONIST REGION y Fig 18.22 & 18.23 on pg 563 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y The problem now was to completely remove the agonist activity in order to obtain compounds with pure antagonist activity. y This meant designing a structure which would differentiate between the agonist and the antagonist binding regions. y At first sight this seemed impossible because both regions appear to involve the same type of bonding THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y Histamine’s activity as an agonist depends on the imidazole ring and the charged amino function, with the 2 groups taking part in hydrogen and ionic bonding respectively. y However, the antagonist activity of the partial agonists described so far also appears to depend on a hydrogen bonding imidazole ring and an ionic bonding guanidine group. y HOWEVER................. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y A distinction could be made between the charged groups. y Structures showing antagonist activity are all capable of forming chelated bonding structures (Slide 176) y This interaction involves two hydrogen bonds between two charged species. y The question arose as to whether it was really necessary for the chelating group to be charged, or more clearly..... y Could a neutral group also chelate to the antagonist region by hydrogen bonding alone? THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y If yes, then it could be possible to distinguish between the agonist and the antagonist region, especially since ionic bonding appeared necessary for agonist binding. y The decision was therefore, to evaluate the consequence of replacing the strongly basic guanidine group with a neutral group capable of interacting with the receptor by 2 hydrogen bonds. y These groups were selected also on the basis of not causing any other significant changes to the other properties of the molecule. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y Thus, in order to study the effect of changing the basic guanidine group with a neutral group, it was necessary to ensure that the new group was as similar as possible to guanidine in terms of size, shape and hydrophobicity. y Several functional groups were tried, but success was ultimately achieved by using a thiourea group. y In fact, the thiourea derivative SK&F91581 proved to be a weak antagonist with no agonist activity. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y Apart from basicity, the properties of the thiourea group were very similar to those of the guanidine group. y Both groups were planar, similar in size, and capable of participating in hydrogen bonding. y Thus the alteration in biological activity could be reasonably attributed to the differences in basicity between the two groups. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y Unlike guanidine, the thiourea group is neutral. y This is due to the C=S group which has an electron withdrawing effect on neighbouring nitrogens making them non-basic and more like amide nitrogens. y The fact that a neutral group was capable of binding to the antagonist region and not to the agonist site was taken to imply that the agonist binding region required ionic bonding, and that the antagonist binding region required hydrogen bonding. BURIMAMIDE THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y FROM PARTIAL AGONIST TO ANTAGONIST- BURIMAMIDE DEVELOPMENT: y Further chain extension and the addition of an N-methyl group led to burimamide which was found to have enhanced activity. y These results suggested that chain extension served to move the thiourea group closer to the antagonist binding region, and that addition of the N-methyl group resulted in a beneficial increase in hydrophobicity. y Burimamide is a highly specific competitive antagonist of histamine at H2-receptors and is 100x more potent than Nαguanylhistamine. y Its discovery finally proved the existence of the H2-receptor THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y Despite apparent success, burimamide was not suitable for progression to clinical trials because its antagonist activity was still too low for oral administration. y Attention was next turned to the imidazole ring of burimamide and to the possible tautomeric forms of this ring. y It was argued that if one particular tautomer was preferred for binding to the H2 receptor, then activity could be enhanced by modifying the burimamide structure to favour that tautomer. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y At pH 7.4 it is possible for the imidazole ring to equilibrate between the 2 tautomeric forms I and II via the protonated intermediate III shown on the next slide. y The necessary proton for this process is supplied by water or by an exchangeable proton on a suitable amino acid residue in the binding region. y If the exchange is slow, it is possible that the drug will enter and leave the receptor at a faster rate than the equilibration between the 3 tautomeric forms. IMIDAZOLE RING CAN EQUILIBRATE BETWEEN VARIOUS TAUTOMERIC FORMS THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y And if this latter hypothesis is correct, then the preferred tautomer in a strong agonist such as histamine should also be the preferred tautomer for a strong antagonist. y The graphic (2 slides previous) indicated that the imidazole ring can exist as one ionised and 2 unionised tautomers. y It was thus necessary to consider the likelihood of the preferred tautomer being ionised or otherwise. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y And if this latter hypothesis is correct, then the preferred tautomer in a strong agonist such as histamine should also be the preferred tautomer for a strong antagonist. y The graphic (2 slides previous) indicated that the imidazole ring can exist as one ionised and 2 unionised tautomers. y It was thus necessary to consider the likelihood of the preferred tautomer being ionised or otherwise. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y It has already been seen that the pKa for the imidazole ring in histamine is 5.74, meaning that the ring is a weak base, and mostly unionised. y The pKa value for imidazole itself is 6.80, and for burimamide 7.25. y These values show that these imidazole rings are more basic than histamine, and more likely to be ionised. y The question is why this is so.................. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y This may be explained through consideration of the side chain, which must have an electronic effect on the imidazole ring. y If the side chain is electron withdrawing, or electron donating, then it will affect the basicity of the ring. y A measure of the side chain’s electronic effect can be worked out by the Hammett equation...... THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y pKa(R) = pKa(H) + ρσR y Where pKa(R) is the pKa of the imidazole ring bearing the side chain R, y pKa(H) is the pKa of the unsubtituted imidazole ring y ρ is a constant y And ρ(R) is the Hammett substituent constant for the side chain R. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y From the pKa values, the value of the Hammett substituent constant can be calculated to show whether the side chain R is electron withdrawing or donating. y In burimamide, the side chain was calculated to be slightly electron donating- of the same order of a methyl group. y Therefore, the imidazole ring in burimamide is more likely to be ionised than that in histamine in which the side chain is electron withdrawing. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y At pH 7.4, 40% of burimamide is ionised in the imidazole ring, compared to 3% of histamine. y This represents quite a difference between the 2 structures, and since the binding of the imidazole ring is important both for antagonist and agonist activity, the implication is that a pKa value closer to that of histamine might lead to better binding and to better antagonist activity. y It was necessary therefore, in this drug design project to make the side chain electron withdrawing rather than donating. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y This may be done by inserting an electronegative atom into the side chain- which also has a minimal effect on the rest of the molecule. y In other words, an isostere for a methylene group was sought,one that had the desired electronic effect, but which also had the same size and properties as the methylene group. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y The first isostere to be tried was a sulfur atom. y Sulfur is quite a good isostere for a methylene unit, in that both groups have similar van der Waals radii and similar bond angles. y However, a C-S bond is slightly longer than a C-C bond, leading to a light extension (15%) of the structure. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y The methylene group replaced was next but one to the imidazole ring. y This site was chosen, not for any strategic reasons, but because a synthetic route was readily available to cary out this transformation. y As hoped, the resulting compound, thiaburimamide, had a significantly lower pKa of 6.25, and was found to have enhanced antagonistic activity. y This result supported the theory that a reduction in the proportion of ionised tautomer was beneficial to receptor binding and antagonist activity. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y Thiaburimamide had been synthesised in order to favour the unonised imidazole ring over the ionised ring. y But as previously demonstrated, there are 2 possible unionised tautomers. y The question consequently arose as to whether either of these was preferred for receptor binding. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y In order to answer this question, histamine was once again considered. y If one of the unionised tautomers was found to be preferred over the other in histamine, then the reasonable assumption would be that this is favoured tautomer for receptor binding. y The preferred tautomer for histamine is tautomer 1 y Why is tautomer 1 favoured?................. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y The answer lies in the fact that the side chain on histamine is electron withdrawing. y This electron withdrawing effect on the imidazole ring is inductive, and therefore the strength of the effect decreases the distance around the ring. y The implication is that the nitrogen atom on the imidazole ring closest to the side chain (Nπ) experiences a greater electron withdrawing effect than the one further away (Nτ). y As a result, the closer nitrogen is less basic, which in turn means that it is less likely to bind to hydrogen. y Since the side chain in thiaburimamide is electron withdrawing, then it too will favour tautomer 1. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y It was now argued that this tautomer could be further enhanced if an electron donating group was placed at position 4 in the ring. y At this position, the inductive effect would be felt most at the neighbouring nitrogen (Nτ), further enhancing its basic character and increasing the population of tautomer 1. y It was also important to choose a group which would not interfere with the normal receptor binding interaction. y For example, a large substituent would be too bulky and prevent the analogue fitting the receptor. y A methyl group was chosen since it was known that 4mthylhistamine was an agonist, and was highly selective for the H2 receptor. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y METIAMIDE DEVELOPMENT: y The compound obtained was metiamide which was found to have enhanced activity as an antagonist, supporting the previous theory. y Compared to burimamide, the percentage of ionised imidazole ring was lowered in metiamide, and the ratio of the two possible unionised imidazole tautomers reversed. y The fact that activity is increased with respect to thiaburimiamide suggests that the increase in the population of tautomer 1 outweighs the increase in population of the ionised tautomer 111 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y 4-METHYLBURIMAMIDE DEVELOPMENT: y 4-methylburimamide was also synthesised for comparison. y Here, the introduction of the 4-methyl group did not lead to an increase in activity. y The pKa of 4-methylburimamide is high- 7.80, resulting in the population of the ionised imidazole ring to rise to 72% y This demonstrates the importance of rationalising structural changes- adding a 4-methyl group to thiaburimamide is advantageous, but adding it to burimamide is not. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y The design and synthesis of metiamide followed a rational approach aimed at favouring one specific tautomer in an approach known as dynamic structure activity analysis. y But strangely enough........... THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y It has since transpired that the improvement in antagonism may have also resulted from conformational effects. y X-ray crystallography studies have indicated that the longer thioester linkage in the chain increases the flexibility of the side chain and that the 4- methyl substituent in the imidazole ring may help to orientate the imidazole ring correctly for receptor binding. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y It is significant that the oxygen analogue oxaburimamide was less potent than burimamide despite the fact that the electron withdrawing effect of the oxygen containing chain on the ring is similar to the sulfur containing chain. y The bond lengths and angles of the ether link are similar to the methylene unit, and in thisrespect is a better isostere than sulfur. y However, the oxygen atom is substantially smaller. y It is also significantly more basic and more hydrophilic than either sulfur or methylene. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Oxaburimamide’s lower activity might be due to a variety of reasons. y For example, the oxygen may not allow the same flexibility permitted by the sulfur atom. y Alternatively, the oxygen may be involved in a hydrogen bonding interaction either with the receptor or with its own imidazole ring resulting in a change in receptor binding interaction. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRATEGY: y Metiamide is 10x more active than burimamide, and showed promise as an anti-ulcer agent. y Unfortunately, a number of patients suffered kidney damage and granulocytopaenia- a condition which results in the reduction of circulating white blood cells, and makes patients susceptible to infection. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y DEVELOPMENT OF CIMETIDINE: y It was proposed that metiamide’s side effects were associated with the thiourea group- a group which is not particularly common in the body’s biochemistry. y Therefore consideration was given to replacing this group with a group which was similar in property, but would be more acceptable in a biochemical context. The urea analogue was tried but was found to be less active CHEMICAL STRUCTURE OF THE GUANIDINE ANALOGUE y The guanidine analogue (top) was also less active, but it was interesting to note that this compound had no agonist activity. y This contrasts with the 3-carbon bridged guanidine (below) which has already been shown to be a partial agonist. y This made the guanidine analogue (top) the first example of a guanidine having pure antagonist activity. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y DEVELOPMENT OF CIMETIDINE: y One possible explanation for this is that the longer 4-unit chain extends the guanidine binding group beyond the reach of the agonist binding region, whereas the shorter 3unit chain still allows binding to both agonist and antagonist regions. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design 4‐Carbon Unit Chain 3‐Carbon Unit Chain y Fig 18.33 y Fig 18.34 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y DEVELOPMENT OF CIMETIDINE: y The antagonist activity for the guanidine analogue was weak, but consideration was given to this compound since it was thought that the guanidine unit would be less likely to have toxic side effects than the thiourea. y This was a reasonable assumption since the guanidine unit is naturally present in the amino acid arginine. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y DEVELOPMENT OF CIMETIDINE: y The problem now was to retain the guanidine unit but to increase activity. y It seemed likely that the low activity was due to the fact that the basic guanidine group would be ionised at pH7.4. y The problem was how to make this group neutral- no easy task- considering that guanidine is one of the strongest bases in organic chemistry. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y DEVELOPMENT OF CIMETIDINE: y Nevertheless, a search of the literature revealed a useful study on the ionisation of monosubstituted guanidines. y A comparison of pKa values of these compounds with the inductive substituent complexes σi for the substituents X yielded a straight line indicating that pKa is inversely proportional to the electron withdrawing power of the substituents. y Thus, strongly electron withdrawing substituents make the guanidine group less basic and less ionised. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design Ionisation of monosubstituted guanidines y Fig 18.36 pKa vs Inductive Substituent Constants (σi) for X on the LHS y Fig 18.37 THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y DEVELOPMENT OF CIMETIDINE: y Both the nitroguanidine and cyanoguanidine analogues of metiamide were synthesised and found to have comparable antagonist activities to metiamide. y The cyanoguanidine analogue was cimetidine, and was the more potent analogue that was chosen for clinical studies THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y BIOLOGICAL ACTIVITY OF CIMETIDINE: y Cimetidine inhibitsed H2 receptors and consequently also inhibited gastric acid release. y It did not have the toxicity of metiamide, and was also more potent than metiamide. y It also inhibited pentagastrin from stimilating the release of gastric acid. y Pentagastrin is an analogue of gastrin, and the fact that cimetidine was capable of blocking its stimulatory activity suggested a relationship between histamine and gastrin in the release of gastric acid. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y CIMETIDINE: y Cimetidine was first marketed in the UK in 1976 under the trade name Tagamet (derived from anTAGonist and ciMETidine) y It was the first really effective ant-ulcer drug that successfully did away with the need for surgery, and for several years it was the world’s best selling prescription drug until it was pushed into second place in 1988 by ranitidine. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRUCTURE & ACTIVITY OF CIMETIDINE: y The finding that metiamide and cimetidine were both good H2-antagonists of similar activity shows that the cyanoguanidine group is a good bioisostere for the thiourea group. y This is despite the fact that three tautomeric forms are possible for the guanidine group compared to only one for the isothiourea group. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRUCTURE & ACTIVITY OF CIMETIDINE: y The 3 tautomeric forms of the guanidine unit were foundto be more apparent than real, since the imino tautomer (II) is the preferred tautomeric form for the guanidine unit. y Tautomer II is favoured since the cyano group has a stronger electron withdrawing effect on the neighbouring nitrogen compared to the two nitrogens further away. y This makes the neighbouring nitrogen less basic and less likely to be protonated. THE CIMETIDINE STORY‐ A Rational Approach to Drug Design y STRUCTURE & ACTIVITY OF CIMETIDINE: y Since tautomer II is favoured, the guanidine group does in fact bear a close structural resemblance to the thiourea group. y Both groups have a planar π electron system with similar geometries (equal C-N distances and angles). y They are polar and hydrophilic with high dipole moments and low partition coefficients. y They are weakly basic and weakly acidic such that they are unionised at pH7.4 VARIATION OF THE IMIDAZOLE RING‐ Ranitidine y Ranitidine: y Further studies on cimetidine analogues showed that the imidazole ring could be replaced with other nitrogen containing heterocyclic rings. y However, Glaxo moved one step further by showing that the imidazole ring could be replaced by a furan ring bearing a nitrogen containing substituent. y This led to the introduction of ranitidine CHEMICAL STRUCTURE OF RANTIDINE (ZANTAC®) Ranitidine has fewer side effects than cimetidine, has a longer half life, and is 10x more active. VARIATION OF THE IMIDAZOLE RING‐ Ranitidine y SAR RESULTS FOR RANITIDINE INCLUDE: y The nitroketeneaminal group is optimum for activity, but may be replaced by other planar π systems capable of hydrogen bonding y Replacing the sulfur atom with a methylene group leads to a drop in activity y Replacing the furan ring with more hydrophobic rings such as phenyl or thiophene reduces activity VARIATION OF THE IMIDAZOLE RING‐ Ranitidine y SAR RESULTS FOR RANITIDINE INCLUDE: y 2,5-disubstitution is the best substitution pattern for the furan ring. y Substitution on the dimethylamino group may be varied, showing that the basicity and hydrophobicity of this group are not crucial to activity. VARIATION OF THE IMIDAZOLE RING‐ Ranitidine y SAR RESULTS FOR RANITIDINE INCLUDE: y Methyl substitution at carbon 3 of the furan ring eliminates activity, whereas the equivalent substitution on the imidazole series increases activity. y Methyl substitution at carbon 4 of the furan ring eliminates activity, whereas the equivalent substitution in the imidazole series increases activity. VARIATION OF THE IMIDAZOLE RING‐ Ranitidine y SAR RESULTS FOR RANITIDINE INCLUDE: y These latter 2 results imply that the heterocyclic rings for cimetidine and ranitidine are not interacting in the same way with the H2 receptor. y This is suported by the fact that a corresponding dimethylaminomethylene group attached to cimetidine leads to a drop in activity. y Ranitidine was introduced into the market in 1981, and by 1988 became the world’s best selling prescription drug. FAMOTIDINE & NIZATIDINE y During 1985 and 1987 two new antiulcer drugs were introduced to the market- famotidine and nizatidine. y Famotidine is 30x more active than cimetidine in vitro. y The side chain contains a sulfonylamidine group while the heterocyclic imidazole ring of cimetidine has been replaced with a 2-guanidinothiazole ring FAMOTIDINE & NIZATIDINE y SAR STUDIES ON FAMOTIDINE INDICATE THAT: y The sulfonylamidine binding group is not essential and may be replaced by a variety of structures as long as they are planar, have a dipole moment, and are capable of interacting with the receptor by hydrogen bonding. A low pKa is not essential, which allows a larger variety of planar groups to be used than is possible for cimetidine. y Activity is optimum for a chain length of 4 or 5 units. y Replacement of sulfur with a CH2 group increases activity FAMOTIDINE & NIZATIDINE y SAR STUDIES ON FAMOTIDINE INDICATE THAT: y Modification of the chain is possible with, for example, inclusion of an aromatic ring y A methyl substituent ortho to the chain leads to a drop in activity (unlike the cimetidine series). y 3 of the 4 hydrogens in the 2 NH2 groups are required for activity. y There are several results which are markedly different from cimetidine, implying that famotidine and cimetidine are not interacting in the same way with the H2-receptor FAMOTIDINE & NIZATIDINE y NIZATIDINE: y Nizatidine was introduced into the UK in 1987 by the Lilly Corporation. y It is equipotent to ranitidine y The furan ring in ranitidine is replaced with a thiazole ring H2 ANTAGONISTS WITH PROLONGED ACTIVITY y There is presently need for longer lasting antiulcer agents which require once daily doses. y GSK carried out further development on ranitidine by placing the oxygen of the furan ring exocyclic to a phenyl ring and replacing the dimethylamino group with a piperidine ring to give a series of novel structures. H2 ANTAGONISTS WITH PROLONGED ACTIVITY y The most promising of these compounds were lamitidine and loxitidine which were 5 – 10x more potent than ranitidine and 3x longer lasting. y Unfortunately, these compounds showed toxicity in long term animal strudies, with the possibility of causing gastric ulcer. y They were subsequently withdrawn from clinical study. COMPARISON OF H1 & H2 ANTAGONISTS y The structures of the H2 antagonists are markedly different to the classical H1 antagonists, so there is little surprise that the original antihistamines failed to antagonise the H2 receptor. y H1 antagonists like H1 agonists possess an ionic amino group at the end of a flexible chain. y Unlike the agonists they possess 2 aryl or heteroaryl rings in place of the imidazole ring see 18.62 COMPARISON OF H1 & H2 ANTAGONISTS y Because of the aryl rings, H1 antagonists are hydrophobic molecules having high partition coefficients. y In contrast, H2 antagonists are polar and hydrophilic, having high dipole moments and low partition coefficients. y At the end of the flexible chain they have a polar p electron system which is weakly amphoteric and unionised at pH 7.4 y This binding group appears to be the key feature leading to antagonism of H2 receptors. COMPARISON OF H1 & H2 ANTAGONISTS y The 5- membered heterocycle generally contains a nitrogen atom, or, in the case of furan or phenyl, a nitrogen containing side chain. y The hydrophilic character of H2 antagonists helps to explain why H2 antagonists are less likely to have CNS side effects often associated with H1 antagonists. THE H2 RECEPTOR & H2 ANTAGONISTS y H2 receptors are present in a variety of organs and tissues, but their main role is acid secretion. y As a result, H2 antagonists are remarkably safe, and mostly free of side effects. ANTIBACTERIAL AGENTS y The fight against bacterial infection is one of the great success stories of medicinal chemistry y Bacteria were first identified in the 1670s by van Leeuwenhoek following his invention of the microscope. y It was not until the 19th century that their link with disease was appreciated following the experiments of Pasteur who demonstrated that specific bacterial strains were crucial to fermentation, and that these and other microrganisms were far more widespread than previously thought. ANTIBACTERIAL AGENTS y An early advocate of a germ theory of disease was the Edinburgh surgeon Lister. y Despite the protests of several colleagues who took offence at the suggestion that they might be infecting their own patients, Lister introduced carbolic acid as an antiseptic and sterilising agent for operating theatres and wards. y The improvement in surgical survival rates was significant. ANTIBACTERIAL AGENTS y During the latter half of the 19th century, scientists such as Koch were able to identify the micro-organisms responsible for diseases such as tuberculosis, cholera and typhoid. y Methods such as vaccination for fighting infections were studied. y Research was also carried out to try to find effective antibacterial agents or antibiotics ANTIBACTERIAL AGENTS y Erlich’s Principle of Chemotherapy was that a chemical could directly interfere with the proliferation of microorganisms at concentrations tolerated by the host. y Thus was the notion of selective toxicity, introduced to therapeutics. y This selectivity came to be represented by a chemotherapeutic index which compared the minimum effective dose of a drug with the maximum dose which could be tolerated by the host. y This principle was later extended to all drug classes- hence the widely used term therapeutic index. MECHANISMS OF ANTIBACTERIAL ACTION‐ 5 MAIN MECHANISMS y INHIBITION OF CELL METABOLISM BY ANTIMETABOLITES y They inhibit the metabolism of a microorganism but not of the host y They do this by inhibition of an enzyme catalysed reaction present in the bacterial cell but not in animal cells y The best known examples of antibacterial agents acting in this way are the sulphonamides ANTIBACTERIAL AGENTS y INHIBITION OF CELL WALL SYNTHESIS y This leads to bacterial cel lysis and death. y Agents acting in this way include penicillins & cephalosporins. y Since animal cells do not have a cell wall, they are unaffected by such agents. ANTIBACTERIAL AGENTS y INTERACTION WITH THE PLASMA MEMBRANE y Some antibacterial agents interact with the plasma membrane of bacterial cells to affect membrane permeability. y This has fatal results for the bacterial cell. y Polymyxins and tyrothricin operate in this way ANTIBACTERIAL AGENTS y DISRUPTION OF PROTEIN SYNTHESIS. y This means that essential enzymes required for the bcterial cell’s survival can no longer be made. y Agents which disrupt protein synthesis include the rifamycins, aminoglycosides, tetracyclines & chloramphenicol. ANTIBACTERIAL AGENTS y INHIBITION OF NUCLEIC ACID TRANSCRIPTION & REPLICATION y Inhibition of nucleic acid function prevents cell division and/or the synthesis of essential enzymes. y Agents acting in this way include nalidixic acid and proflavine THE ANTIMETABOLITES‐ the sulphonamides y The sulphonamide story starts in 1935 when it was discovered that a red dye, prontosil rubrum, had in vivo antibacterial properties, when these were adiminstered to laboratory animals. y It was also significant, that prontosil rubrum, could not kill bacteria in a test tube. y This fact remained a mystery until it was discovered that prontosil itself was not the antibacterial agent. THE ANTIMETABOLITES‐ the sulphonamides y Instead, it was found that the dye was metabolised by bacteria present in the small intestine of the test animal, and broken down to give a product called sulphanilamide. y It was this compound which was the true antibacterial agent. y Prontosil thus became the first example of a pro-drug, and sulphanilamide was synthesised in the laboratory and became the first synthetic antibacterial agent, active against a wide variety of infections. Metabolised Prontosil (Pro-Drug) Sulphanilamide METABOLISM OF PRONTOSIL THE ANTIMETABOLITES‐ the sulphonamides y Further developments led to a range of sulphonamides which proved effective against Gram positive organisms specifically meningococci and pneumococci. y Despite their undoubted benefits, sulpha drugs were ineffective against Salmonella- the organism responsible for typhoid. y Other problems resulted from the way in which these drugs are metabolised, since toxic products are frequently obtained. y For this reason, sulphonamides were largely superseded by penicillin THE ANTIMETABOLITES‐ the sulphonamides ‐SAR The synthesis of a large number of sulphonamide analogues (see next slide) led to the following conclusions: yThe para amino group is essential for activity and must be unsubstituted (ie R=H). The only exception is when R is an acyl group ie amides. The amides themselves are inactive, but may be metabolised in vivo to regenerate the active compound (see 2 slides over). This means that amides can be used as sulphonamide prodrugs. SULPHONAMIDE ANALOGUES Metabolism of acyl group to regenerate active compound THE ANTIMETABOLITES‐ the sulphonamides ‐SAR y The aromatic ring and the sulphonamide functional group are both required. y The aromatic ring must be para substituted only y The sulphonamide nitrogen must be secondary y R’’ is the only possible site that can be varied in sulphonamides SULPHANILAMIDE ANALOGUES y R’’ can be varied by incorporating a large range of heterocyclic or aromatic structures which affect the extent to which the drug binds to plasma proteins y This in turn controls the blood levels of the drug such that it can be short acting or long acting y Thus a drug which binds strongly to plasma protein will be released more slowly into the circulation and will be longer lasting SULPHANILAMIDE ANALOGUES y Changing the nature of the R’’ has also helped to reduce the toxicity of some sulphonamides. y The primary amino group of sulphonamides is acetylated in the body and the resulting amides have reduced solubility which can lead to toxic effects. y For example, the metabolites formed from an early sulphonamide, sulphathiazole, (see slide overleaf), is poorly soluble and may prove fatal if it blocks the renal tubule. N-acetylation INSOLUBLE SULPHANILAMIDE ANALOGUES y It was discovered that the solubility problem could be overcome by replacing the thiazole ring in sulphathiazole with a pyrimidine ring to give sulphadiazine. y The reason for the improved solubility lies in the acidity of the solubility lies in the acidity of the sulphonamide NH proton. y In sulphathiazole, this proton is not very acidic (high pKa). y Therefore, sulphathiazole and its metabolite are mostly unionised at blood pH. SULPHANILAMIDE ANALOGUES y Replacing the thiazole ring with a more electron withdrawing pyrimidine ring increases the acidity of the NH proton by stabilising the anion which results. y Therefore, sulphadiazine and its metabolite are significantly ionised at blood pH. y As a consequence, they are more soluble and less toxic. pKa 6.48 86% Ionised SULPHANILAMIDE ANALOGUES‐ Sulphadiazine y Was found to be more active than sulphathiazole, and soon replaced it in therapy. y The corollary therefore is that varying R” can affect the solubility of sulphonamides or the extent to which they bind to plasma protein. y These variations are therefore affecting the pharmacokinetics of the drug rather than its mechanism of action. SULPHANILAMIDES‐The Future y The penicillins largely superseded the sulphonamides in the fight against bacterial infections, and for a long time, sulphonamides were relegated backstage. y There has of late, been a revival of interest with the discovery of a new breed of longer lasting sulphonamides. y One example of this new generation is sulphamethoxine, (see next slide), which is so stable in the body it need only be taken once a week. SULPHA DRUGS‐Current Applications in Medicine y Treatment of Urinary Tract Infections y Ophthalmic Use. y Treatment of Mucous Membrane Infection. y Treatment of Gut Infections EXAMPLES OF OTHER ANTI‐ METABOLITES y There are other antimetabolites in medical use apart from the sulphonamides. y Two examples are trimethoprim and a group of compounds known as sulfones. SULFONE STRUCTURE TRIMETHOPRIM y Is a diaminopyrimidine structure which has proved to be a highly selective, orally active, antibacterial and antimalarial agent. y Unlike the sulphonamides it inhibits dihydrofolate reductase- the enzyme which carries out the conversion of folic acid to tetrahydrofolate. y The overall effect, however, is the same as with the sulphonamides- the inhibition of DNA synthesis and cell growth TRIMETHOPRIM y Dihydrofolate reductase is present in mammalian cells as well as in bacterial cells. y Trimethoprim distinguishes between enzymes in either cell type, owing to the fact that mutations over millions of years have resulted in a significant difference in structure between the two enzymes such that trimethoprim recognises and inhibits the bacterial enzyme, but does not recognise the mammalian enzyme. TRIMETHOPRIM y Is often given in conjunction with the sulphonamide sulphamethoxazole (co-trimoxazole Septrin®) y Sulphamethoxazole inhibits the incorporation of PABA into folic acid; trimethoprim inhibits dihydrofolate reductase. This results in the inhibition of two enzymes in the same biosynthetic route; and is a very effective way of inhibiting a biosynthetic route and has the advantage of keeping the doses of both drugs to sub-toxic levels. y The acquistion of the same level of inhibition using a single drug would require much higher doses, leading to potential side effects. y This approach has been described as SEQUENTIAL BLOCKING H N CO2 H TETRAHYDROFOLATE FOLIC ACID H OMe O H CH 3 N H H S H N N CH N H O 2 N OMe N O SULFAMETHOXAZOLE OMe TRIMETHOPRIM BACTERIAL CELL WALL SYNTHESIS INHIBITORS y There are 2 major classes of drug which act in this fashion y These are the penicillins & the cephalosporins BRIEF HISTORY OF THE PENICILLINS y In 1877, Pasteur & Joubert discovered that certain moulds could produce toxic substances which killed bacteria. Unfortunately, these substances were also toxic to humans and had no clinical value. They demonstrated however, that moulds could be a source of antibacterial agents. y In 1928, Fleming noted that a bacterial culture which had been left open to the air for several weeks had become infected by a fungal colony. Of interest was the fact that there was an area surrounding the fungal colony where the bacterial colonies were dying. y He correctly concluded that the fungal colony was producing an antibacterial agent which was spreading to the surrounding area. BRIEF HISTORY OF THE PENICILLINS y Recognising the significance of this, he set out to culture and identify the fungus, and showed it to be a relatively rare species of Penicillium. y It has since been suggested that the Penicillium spore responsible for the fungal colony originated from another laboratory in the building, and that the spore was carried by air currents, and was eventually blown through the window of Fleming’s laboratory. BRIEF HISTORY OF THE PENICILLINS y Fleming spent several years investigating the novel antobacterial substance, and showed it to have significant anti-bacterial properties, and to be remarkably non-toxic to humans. y Unfortunately, the substance was also unstable, and Fleming was unable to isolate & purify the compound. y He therefore came to the conclusion that penicillin was too unstable to be used clinically. BRIEF HISTORY OF THE PENICILLINS y The problem of isolating penicillin was eventually solved in 1938 by Florey & Chain by using a process known as freeze drying, which allowed isolation of the antibiotic under much milder conditions than had been previously available. y By 1941, Florey & Chain were able to carry out the first clinical trials on crude extracts of penicillin, and achieved spectacular success. y Further developments aimed at producing the new agent in large quantities were developed in the US, such that by 1944 there was enough penicillin for casualties arising from the D-Day landings. BRIEF HISTORY OF THE PENICILLINS y This led to a widespread use of penicillin- however, the structure of the compound remained unresolved. y The issue was finally settled in 1945 when Dorothy Hodgkins established the exact structure (see overleaf) through X-ray analysis. R= Benzyl Penicillin (PEN G) 6-Aminopenicillanic Acid R= Phenoxymethylpenicillin (PEN V) Acyl Side Chain β -Lactam Ring Thiazolidine Ring BRIEF HISTORY OF THE PENICILLINS y The synthesis of such a highly strained molecule presented a huge challenge. This was overcome by Sheenan who completed a full synthesis of the molecule by 1957. y This synthetic pathway was too involved to be of commercial use, but the following year Beechams isolated a biosynthetic intermediate of penicillin. y This revolutionised the field of penicillins by providing the starting material for a huge range of semisynthetic penicillins BRIEF HISTORY OF THE PENICILLINS y Penicillins were widely & carelessly used so that the evolution of penicillin resistant bacteria became more and more of a problem. y The fight against these penicillin-resistant bacteria was promoted greatly when, in 1976 Beechams discovered a natural product called clavulanic acid which has proved highly effective in protecting penicillins from the bacterial enzymes which protect them. Sulphur replaced by Oxygen No acylamino side chain 6 7 9 4 5 8 3 1 2 β -Lactam Oxazolidine Ring THE STRUCTURE OF PENICILLIN y The structure of penicillin is unusual, such that many scientists were not convinced of its veracity until this was proven through X Ray crystallography. y It is comprised of a highly unstable looking bicyclic system consisting of a 4-membered β-lactam ring fused to a 5 membered thiazolidine ring. y The skeleton of this molecule suggests that it is derived from the amino acids cysteine and valine. y The overall shape of the molecule is like that of a half open book: CYS 3 3 2 VAL PENICILLIN APPEARS TO BE DERIVED FROM CYSTEINE & VALINE THE STRUCTURE OF PENICILLIN y The acyl side chain R varies depending on the make up of the fermentation media used during the synthetic process. y For example, corn steep liquor was used as a medium when penicillin was first produced in the United States, and this gave Penicillin G (R = benzyl) y This was due to the high levels of phenylacetic acid (PhCH2CO2H) present in the medium. PENICILLIN ANALOGUES y In 1957, Sheehan succeeded in synthesising penicillin and obtained a 1% yield of penicillin V using a multistep synthetic route. y Clearly full synthesis was not an efficient way of making penicillin analogues. y In 1958-1960, Beechams managed to isolate a biosynthetic intermediate of penicillin which was also one of Sheehan’s synthetic intermediates. PENICILLIN ANALOGUES y The compound was 6-APA and it allowed the synthesis of a huge number of analogues by a semi-synthetic method. y This means that fermentation yielded 6-APA which could then be treated synthetically to give semi-synthetic analogues. y This was achieved by acylating the 6-APA with a range of acid chlorides 2 3 3 3 3 2 PENICILLIN ANALOGUES ACHIEVED BY ACYLATING 6-APA PENICILLIN ANALOGUES y 6-APA is now produced by hydrolysis of penicillin G or V with an enzyme- penicillin acylase or by other chemical methods which will be discussed later on in this unit. y These are more efficient procedures than fermentation. H H N O H H S O C H2 N O C H3 C H3 CO2 H H H N C H2 PRODUCTION OF 6-APA H S N O HYDROLYSIS H C H3 C H3 CO2 H