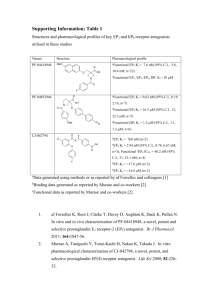
Appendix 1
advertisement

On line data supplement 2010_158568_Belvisi
EP4 receptor as a new target for bronchodilator therapy
James Buckley1, Mark A Birrell1, Sarah A Maher1, Anthony T Nials2
Deborah L Clarke1 & Maria G Belvisi1
1
Respiratory Pharmacology, Pharmacology & Toxicology Section, Imperial College London,
Faculty of Medicine, National Heart and Lung Institute, Sir Alexander Fleming Building,
London, SW7 2AZ, UK; 2Respiratory CEDD. GlaxoSmithKline Research and Development,
Medicines Research Centre, Gunnels Wood Road, Stevenage, Hertfordshire SG1 2NY, UK
Correspondence:
Professor Maria G. Belvisi , Phone: +44 (0)20 7594 7828, Fax: + 44
(0)20 7594 3100, e-mail: m.belvisi@imperial.ac.uk
METHODS
Animals
Male C57BL/6 mice (18-20 g), Dunkin-Hartley guinea pigs (300-500 g) and Cynomolgus
(Cyno) monkey (Macaca fascicularis) tissue were purchased from Harlan (Bicester, Oxon,
UK). Male Sprague Dawley rats (250-275g) were purchased from Charles River (Margate,
UK). EP1, EP2 and EP3, IP, FP and DP1 gene deficient mice were derived from breeding
pairs devoid of the Ptger 1, Ptger2, Ptger3, Ptgir, Ptgfr and Ptgdr genes respectively, that had
been backcrossed at least eight times onto the C57/BL/6 background. Ptger4 -/- mice are non
viable on the C57BL/6 background due to patent ductus arteriosus20 and were therefore
backcrossed onto a mixed background of 129/Ola X C57BL/6.
Mice were originally
provided by Dr. Shuh Narumiya, Kyoto University, and breeding colonies were maintained at
Imperial College, London. All experiments were conducted in accordance with UK Home
Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986.
Preparation of samples
Guinea pigs, mice and rats were euthanized via an overdose of sodium pentobarbital
(200mg/kg, i.p.). Human airway samples (trachea, major bronchus, secondary bronchi) were
obtained from donor patients (n=7, 3 male) or recipients (emphysema, 2 male, n=3: cystic
fibrosis, n=1 male) for lung transplants performed at The Royal Brompton or Harefield
Hospital. Approval was obtained from the Royal Brompton and Harefield ethics committee
after receiving the relevant consent from patients and relatives. The airway from all species
was carefully dissected and placed in Krebs-Henseleit (KH) solution (composition in mM:
NaCl 118, KCl 5.9, MgSO4 1.2, CaCl2 2.5, NaH2PO4 1.2, NaHCO3 25.5, glucose 5.6)
containing indomethacin (to prevent the formation of endogenous prostanoids), maintained
at room temperature and continuously bubbled with 95% O2/ 5% CO2. Trachea (apart from
murine) was opened longitudinally by cutting through the cartilage directly opposite to the
smooth muscle layer. Unless stated otherwise, the epithelium was left intact and transverse
segments were prepared and sutured in order to be suspended from steel hook transducers in
10ml baths containing KH solution warmed to 37˚C. Where the epithelium was removed,
after opening the trachea transversely, a wet cotton bud was used to gently rub the surface of
the tissue and remove the epithelial cells. Following the experiment, any such tissues were
kept in formalin for histological confirmation that epithelium cells had been removed.
Murine trachea was dissected into two equal segments by cutting transversely through the
longitudinal midpoint and left unopened; two rings of suture were tied around opposite sides
of the airway, which were then connected to the transducers as described above. Tissues
were left to equilibrate under a resting tension of 1 g (guinea pig, rat, monkey) or 0.8 g
(mouse) or 2g (human) for at least 60 minutes with regular (every 20 minutes) washing
before beginning the experiment. Changes in force were measured isometrically using forcedisplacement transducers (model FT-03c, Grass Instrument, Quincy, MA, U.S.A.) connected
to a data acquisition system (MP100) operating on a Windows PC using AcqKnowledge
software (BIOPAC systems, U.S.A) as previously described21. Following equilibration the
contractile response was assessed in each tissue using a supramaximal concentration of
acetylcholine (ACh; 10 mM).
Protocol for experiments conducted on basal tone
After washout and once a stable basal tone was established, any antagonists/vehicles used in
the experiment were administered to the relevant baths and incubated for 30 minutes.
Cumulative concentration response curves were subsequently constructed using the relevant
contractile agents as previously described22.
Protocol for experiments conducted in induced tone
Carbachol (CCh; 1 µM) was used to induce increased tension in tissues over a 20 minute
period before the addition of any antagonists or their appropriate vehicles which were
incubated for a further 30 minutes. Cumulative concentration response curves were then
constructed using the relevant drugs and subsequently, at the end of the experiment the non
specific phosphodiesterase inhibitor, papaverine (100 µM), was used to assess the maximum
capacity for relaxation of each tissue.
Data analysis and Statistics
Data are expressed as mean ± SEM of n independent observations. Concentration response
curves were analysed by non-linear regression using the “PRISM” curve-fitting program
(Graph-Pad software, CA, U.S.A.) to produce curves of best fit, from which EC50 values were
subsequently derived. Estimates of antagonist affinity were calculated using the equation pKB
= log (CR-1) – log [B] as described by Gaddum (1957)23, where CR is the concentration ratio
calculated from the EC50 of agonist in the presence of the antagonist divided by the EC50 of
the agonist alone, KB is the equilibrium dissociation constant, and [B] is the concentration of
antagonist. In the experiments described herein the term pA2 is substituted for pKB, as
antagonists were used at one concentration only, which precludes assumptions being made
about the nature of the antagonism.
Compounds and Materials
Antagonists: The EP1 antagonist GW848687X24 and EP4 antagonist GW627368X25 were
received as a gift from GlaxoSmithKline. The EP4 antagonist ONO-AE3-20826 was received
as a gift from ONO pharmaceuticals (Japan). The EP3 antagonist L-826266 was received as a
gift from Merck Frosst 27, the IP antagonist RO3244794 was received as a gift from Roche
Palo Alto (Palo Alto, CA)28 and the EP1/EP2/DP antagonist AH680929 and FP antagonist
AL-881030 were purchased from Cayman Europe (Tallinn, Estonia). All antagonists were
reconstituted using DMSO and 1-10 mM stocks were stored at -20˚C until required.
Agonists: The EP1-4 agonists ONO-D1-004, ONO-AE1-259, ONO-AE-248 and ONO-AE132931, 32 were received as gifts from ONO pharmaceuticals (Japan) and stock solutions of 1
mM made in 10% DMSO in Krebs.
AH1320516 was received as a gift from
GlaxoSmithKline. PGE2 was purchased from Cayman Europe and stock solutions of 10mM
made in ethanol. Isoprenaline and papaverine were purchased from Sigma Aldrich (Poole,
UK) and dissolved in distilled water at 1 mM and 100 mM respectively. AH1320516 was also
purchased from Sigma Aldrich and stock solutions of 10 mM made in ethanol.
Other compounds: Krebs salts were obtained from BDH (Dorset, UK) and all other
chemicals and reagents were purchased from Sigma Aldrich. Acetylcholine and carbachol
were purchased from Sigma Aldrich and dissolved in KH solution at 1 M and 1 mM
respectively.
REFERENCES
S1. Segi, E, Sugimoto, Y, Yamasaki, A, et al. Patent Ductus Arteriosus and Neonatal Death
in Prostaglandin Receptor EP4-Deficient Mice. Biochemical and Biophysical Research
Communications 1998; 246: 7-12.
S2. Van Rossum JM. Cumulative dose-response curves. Arch Int Pharmacodyn 1963; 143:
299-330.
S3. Gaddum JH. Theories of drug antagonism. Pharmacol. Rev. 1957; 9: 211-218.
S4. Giblin, GMP, Bit, RA, Brown, SH et al. The discovery of 6-[2-(5-chloro-2-{[(2, 4difluorophenyl)
methyl]
oxy}
phenyl)-1-cyclopenten-1-yl]-2-pyridinecarboxylic
acid,
GW848687X, a potent and selective prostaglandin EP1 receptor antagonist for the treatment
of inflammatory pain. Bioorganic & medicinal chemistry letters, 2007; 17: 385-389.
S5. Wilson, RJ, Giblin, GMP, Roomans, S et al GW627368X ((N-{2-[4-(4, 9-diethoxy-1oxo-1, 3-dihydro-2H-benzo [f] isoindol-2-yl) phenyl] acetyl} benzene sulphonamide): a
novel, potent and selective prostanoid EP4 receptor antagonist. British journal of
pharmacology, 2006; 148: 326-329.
S6. Kabashima, K, Saji, T, Murata, T et al. The prostaglandin receptor EP4 suppresses colitis,
mucosal damage and CD4 cell activation in the gut. Journal of Clinical Investigation, 2002;
109: 883-894.
S7. Gallant M, Carriere MC, Chateauneuf A, et al. Structure activity relationship of biaryl
acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg Med Chem Lett
2002;12: 2583-2586
S8. Bley KR, Bhattacharya A, Daniels DV et al. RO1138452 and RO3244794:
characterization of structurally distinct potent and selective IP (prostacyclin) receptor
antagonists. Br J Pharmacol 2005; 147: 335-345
S9. Keery, RJ & Lumley, P. AH6809, a prostaglandin DP-receptor blocking drug on human
platelets. British journal of pharmacology, 1988; 94: 745.
S10. Griffin BW, Klimko P, Crider JY, et al.. AL-8810: a novel prostaglandin F2α analog
with selective antagonistic effects at the prostaglandin F2α (FP) receptor. J Pharmacol Exp
Ther 1999; 290: 1278-1284
S11. Kiriyama, M, Ushikubi, F, Kobayashi, T et al. Ligand binding specificities of the eight
types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary
cells. British journal of pharmacology, 1997; 122: 217-224.
S12. Suzawa, T, Miyaura, C, Inada, M et al. The role of prostaglandin E receptor subtypes
(EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the
respective EPs. Endocrinology, 2000; 141: 1554-1559.
S13. Nials AT, Vardey CJ, Denyer LH, et al. AH13205, A selective prostanoid EP2 -receptor
agonist. Cardiovascular Drug Reviews, 1993; 11: 165-179.