Anti-Ulcer Agents
advertisement

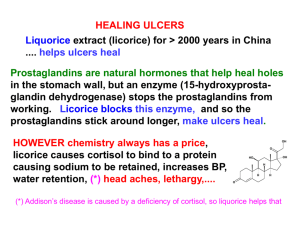
Anti-Ulcer Agents Michael Alwan November 11, 2004 Medicinal Chemistry. Southern Methodist Univ. What is Peptic Ulcer? An Ulcer is … Localized erosion in stomach or duodenum Symptoms and Causes What are the symptoms of a peptic ulcer? Burning pain in the gut Starts 2/3 hours after meals, or in the middle of the night What causes peptic ulcers? Non-Steriodal Anti-Inflammatory Drugs (NSAIDS) Helicobacter pylori Rational Approach to Drug Design Histamine 2 Receptors Proton Pump Inhibitors Tagamet, Zantac, Pepcid, Axid Protonix, Prilosec, Prevacid, Aciphex, Nexium Antibiotics Clarithromycin, Amoxycillan, Tetracyclin H2 Receptor Histamine receptor on parietal cells Autonomic system: food stimulates gastrin release, gastrin stimulates ECL cells, stimulates histamine release, histamine stimulates parietal cells secretion of HCl 2 histamine receptors? If histamine stimulates acid secretion why do antihistamines fail to inhibit other actions of histamine? The possibility of a second histamine receptor … H2 Receptor Antagonist Must bind but not activate H2 receptor site Addition of a functional group to bind with another binding region and prevent the conformational change Addition of aromatic ring: unsuccessful Addition of non-polar, hydrophobic substituents, none antagonists, but … 4-methylhistamine Not an antagonist, but highly H2 selective Conformational isomers show preferential binding 4-methylhistamine Conformation I 4-methylhistamine Conformation II Na -Guanylhistamine First partial agonist First signs of antagonistic activity Still allows partial conformational change Na -Guanylhistamine Guanidine present in A.A. residue arginine Carbon chain lengthened Two-carbon chain, speculation of a carboxylate binding region Three-carbon chain, speculation of different binding region Burimamide Enhanced antagonist activity Longer chain allows for proximity to binding region Terminal methyl group increases hydrophobicity Burimamide Imidazole Ring Development Two tautomers possible, protonation on alternating nitrogens through inductive effects Enhance basicity: addition of electron donating group Decrease basicity: addition of electron withdrawing group Metiamide Cimetidine (Tagmet®) Metiamide is toxic Nitroguanidine and Cyanoguanidine showed similar antagonistic activity NO2>CN>OMe>CONH2>Ac>Ph>H Cimetidine (anTAGonist ciMETidine) Rantidine (Zantaz®) Replace imidazole ring with furan ring 10x more active than Cimetidine Rantidine Famotidine (Pepcid®) 30x more active than cimetidine Famotidine Nizatidine (Axid®) Nizatidine Proton Pump H+/K+ ATPase F-ATPase: in mitocondria and chloroplasts; make ATP with proton gradient V-ATPase: (vacuolar) hydrolyze ATP to generate electrochemical gradient “Proton-Pump” ATPase Animations Proton Pump Inhibitors Exist in inactive form - “prodrugs” Readily converted into active form under low pH Become thiol-reactive: sulfenic acid or cyclosulfenamide Intramolecular rearrangment Inhibitory Mechanism of PPIs PPIs in clinical use Rabeprazole Esomeprazole Mg Lansoprazole Pantoprazole Omeprazole PPI Kinetic Data Omeprazole UV spectra Helicobacter pylori Naturally found in stomach of many people Can cause inflammation; leading to membrane erosion Treated with variety of antibiotics Clarithromycin, Amoxycillan, Tetracyclin Current Treatment Treatment H2 anatagonist / PPI Antibiotic against Helicobacter Pylori Future Increase activity, long-lasting effects Questions?