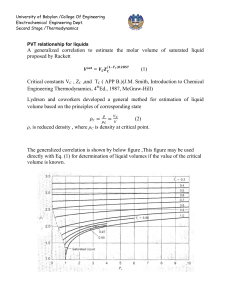
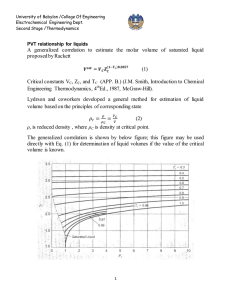
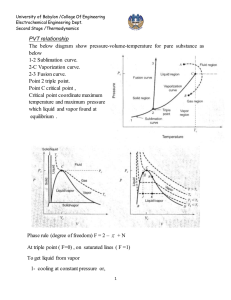
University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics
advertisement

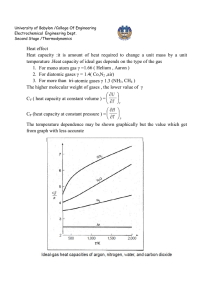
University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Heat effect Heat capacity :it is amount of heat required to change a unit mass by a unit temperature .Heat capacity of ideal gas depends on the type of the gas 1. For mono atom gas γ =1.66 ( Helium , Aaron ) 2. For diatomic gases γ = 1.4( Co,N2 ,air) 3. For more than tri-atomic gases γ 1.3 (NH3, CH4 ) The higher molecular weight of gases , the lower value of γ U CV ( heat capacity at constant volume ) = T V H CP (heat capacity at constant pressure ) = T P The temperature dependence may be shown graphically but the value which get from graph with less accurate University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics On the other hand temperature dependence usually given by an empirical equation; the two simplest expressions of practical value are: CP C T T 2 and , P a bT cT 2 R R Where α , β and , γ and a , b , and c are constants characterized of particular gas , by combine the above two equations : CP D A BT CT 2 2 ( 7-1 ) R T Where C or D is zero , depending on the gas considered .Value of A,B,C and D are CP given in table 4.1,since dimensionless , the unit of C P is governed by choice R of R unit .equation (7-1) used for all gases as well as ideal gas. More accurate but more complex equations are found in literatures. CP = CV + R CV C P 1 R R CV CP , is readily found from equation R R CP CV Effect of temperature on , and are determined by experiment, most often R R from spectroscopic data and knowledge of molecular structure . CP How to use the equation R T2 T2 D H C P dT R A BT CT 2 2 dT T T1 T1 B C 1 1 R[ A(T2 T1 ) (T22 T12 ) (T23 T13 ) D( ) 2 3 T2 T1 Thus the temperature dependence of EX: The molar heat capacity of methane in the ideal gas state is given in table 4.1as CP 1.702 9.081 10 3 T 2.164 10 6 T 2 R CP Where T in Kelvin , develop an equation for for temperature in ○C R University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Solution TK = t ○C +273.15 CP 1.702 9.081 10 3 (t 273.15) 2.164 10 6 (t 273.15) 2 R CP 4.021 7.899 10 3 t 2.164 10 6 t 2 R EX: Calculate the heat required to raise the temperature of 1 mol of methane from 260 to 600 ○C in flow process at constant pressure approximately at 1 bar . Solution T 2873.15 T2 C Q H P dT R T1 ( 1.702 9.081 10 3 T 2.164 10 6 T 2 )dT T 1533.15 Q 2378.8R 2378.8 * 8.314 19780 J As a matter convenience , we define a mean heat capacity T2 Cpmean CpdT T1 T2 T1 When equation (7-1) written by use mean heat capacity equation Cpmean c D 2 A BTam (4Tam T1T2 ) R 3 T1T2 ( 7-2 ) Where Tam= (T1+T2) / 2 is the arithmetic mean temperature. The general equation for all gases and ideal gas EX: Rework the last example by applying equation (7-2) Tam = (533.15 +873.15) / 2 = 703.15 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Cpmean 2.164 10 6 1.702 9.081 10 3 * 703.15 [4 * (703.15) 2 R 3 (533.15) * (873.15)] 6.997 Q = ∆ H = 6.997*8.314*(873.15-533.15) = 19780 J For calculation of T 2 in case given T 1 and Q , used try and error to find T 2 by T2 H T1 Cpmean (7-3) Assume value of T 2 for calculation Cp mean by use equation (7-2) substitution of resulting value into equation (7-3) provides a new value of T 2 which reevaluate Cpmean. Iteration continues to convergence on T 2 value. Heat capacities of solid by used table 4.2 , while heat capacities of liquids from table 4.3. Heat capacity for mixture Cpmean Cpi yi Cpmean( a ) y( a ) Cpmean(b ) y(b ) .... Where : y mole fraction