Document 12643475

University of Babylon /College Of Engineering
Electrochemical Engineering Dept.
Second Stage /Thermodynamics
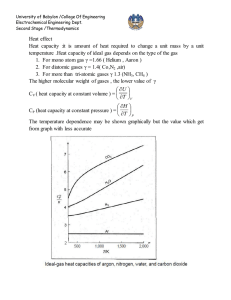
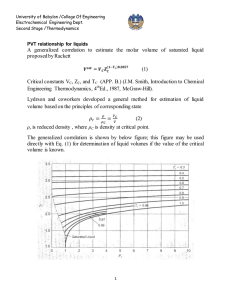
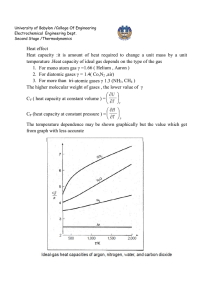
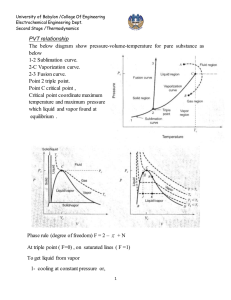
Effect of temperature on standard heat of reaction
Estimation of heat of reaction when reactants and products are not at standard state (298.15
○
K) but on T
○
K
N
2
+ 3 H
2
→ 2NH
3
ν N
2
= - 1 ; ν H
2
= - 3 ; and ,ν NH
3
= 2
i
i
i
f i
4 HCl
( g )
O
2 ( g )
2 H
2
O
( g )
2 Cl
( g )
2
fH
2
O
4
fHCl
( 2 )(
241 , 818 )
( 4 )(
92 , 302 )
114 , 408 J
For standard reactions, products and reactants are always at the standard -state pressure of 1 bar. Standard-state enthalpies are therefore functions of temperature only, and by Eq. d
i
C
Pi dT i identifies a particular product or reactant , multiplying by ν i where , ν
d i d
i
(
i
i
) i i ,
positive ( + ) for products and , negative ( - ) for reactants
d i
C
Pi i
i dT
i
i i
C
Pi dT aA+bB→cC+dD
T d
2 9 8 .
1 5
T
298 .
15
C
P
dT
This is the fundamental equation relating heats of reaction to temperature .
Integrations gives ;
T
298 .
15
C
Pmh
( T
298 .
15 ) ( 1)
University of Babylon /College Of Engineering
Electrochemical Engineering Dept.
Second Stage /Thermodynamics
If the temperature dependence of heat capacity of each product and reactant then
C
Pmh
is given by the analog of equation :
Cp mean
R
A
(
B ) T am
3
C
( 4 T 2 am
T
1
T
2
)
D
T
1
T
2
Where :
∆A≡∑ν i
A i with analogous definitions for
∆B,∆C
, and
∆D
.
Ex -1 : Calculate the standard heat of the methanol –synthesis reaction at 800
○
C
CO
( g )
2 H
2 ( g )
CH
3
OH
By use table 4-4 we can calculate heat of reaction at 25
( g
○
)
C , as below
298
200 , 660
(
110 , 525 )
90 , 135 J
From table 4-1 , take constants value for heat capacity equation i
ν i
A B×10
3
C×10
6
D×10
-5
CH
3
OH 1 2.211 12.216 -3.450 0.000
CO -1 3.376 0.557 0.000 -0.031
H
2
-2 3.249 0.422 0.000 0.083
∆A= (1)(2.211)+(-1)(3.376)+(-2)(3.249) = - 7.663
∆ B= 10.815×10
-3
∆C= -3.450× 10 -6
∆D = -0.135 × 10 -5
By use T1= 298
○
K, T2 = 1073
○
C and R = 8.314 J mol
C
Pmh
17 .
330 J
K
1
-1 ○
K
-1
298
90 , 135
( 17 .
330 )( 1073
298 )
103566 J
J- factor method
The alternative for the equation (1) is general integration to give
T
Cp
J
Eliminating
C
P
dT
C
P
from equation (2) by used the below equation
A
(
B ) T
CT 2
D
R T
And this lead to get the below
T
J
R [
AT
2
B
T 2
C
3
T 3
D
]
T
University of Babylon /College Of Engineering
Electrochemical Engineering Dept.
Second Stage /Thermodynamics
Ex -2 : For the reaction; N
2
+ 3 H
2
→ 2NH
3
298
92220 J i
N
2
H
2
NH
3
ν i
-1
-3
2
A
3.28
3.249
3.578
B×10
3
0.593
0.422
3.020
C×10
6
0
0
0
D×10
-5
0.04
0.083
-0.186
∆A=-5.871
∆ B= 4.181×10 -3
∆C= 0.0
∆D = -0.711×10 5
92220
J
J
8 .
314 [
5 .
871
878151 .
267
298
4 .
181
10
3
2
( 298 )
2
0 .
711
10 5
298
]
T
878151 .
267
8 .
314 [
5 .
871 T
4 .
181
10
3
2
T
2
0 .
711
10 5
]
T
Ex -3 : Repeat Ex-1 by use J- factor method
CO
( g )
2 H
2 ( g )
CH
3
OH
( g )
T
J
R [
AT
B
T 2
2
C
T 3
3
D
]
T
298
90 , 135 J
90 , 135
J
8 .
314 [(
7 .
663 )( 298 )
10 .
815
10
3
2
( 298
0 .
135
10
298
1073
5
J
75259
75259
63710
1073
44 .
962
10
3
298
2
) 2
3 .
450
3
10
9 .
561
10
6
6
( 298
298
3
) 3
1 .
122
10
5
298
1073
103566 J