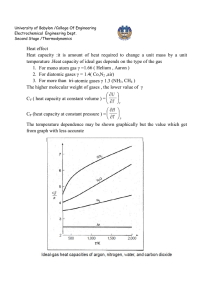
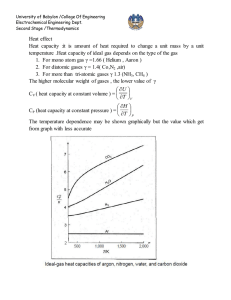
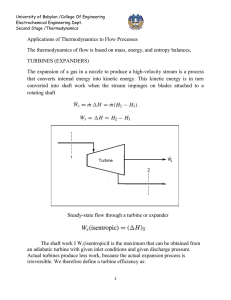
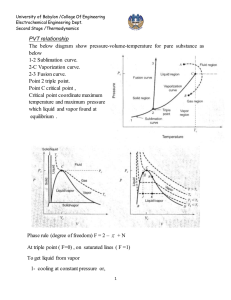
University of Babylon /College Of Engineering Electrochemical Engineering Dept.
University of Babylon /College Of Engineering
Electrochemical Engineering Dept.
Second Stage /Thermodynamics
PVT relationship for liquids
A generalized correlation to estimate the molar volume of saturated liquid proposed by Rackett
(1)
Critical constants V
C
, Z
C
,and T
C
( APP B.)(J.M. Smith, Introduction to Chemical
Engineering Thermodynamics, 4 th
Ed., 1987, McGraw-Hill)
Lydrson and coworkers developed a general method for estimation of liquid volume based on the principles of corresponding state
(2)
ρ r
is reduced density , where
ρ
C
is density at critical point.
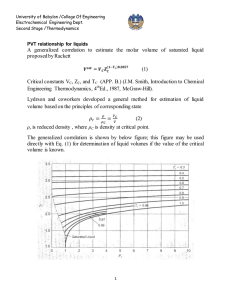
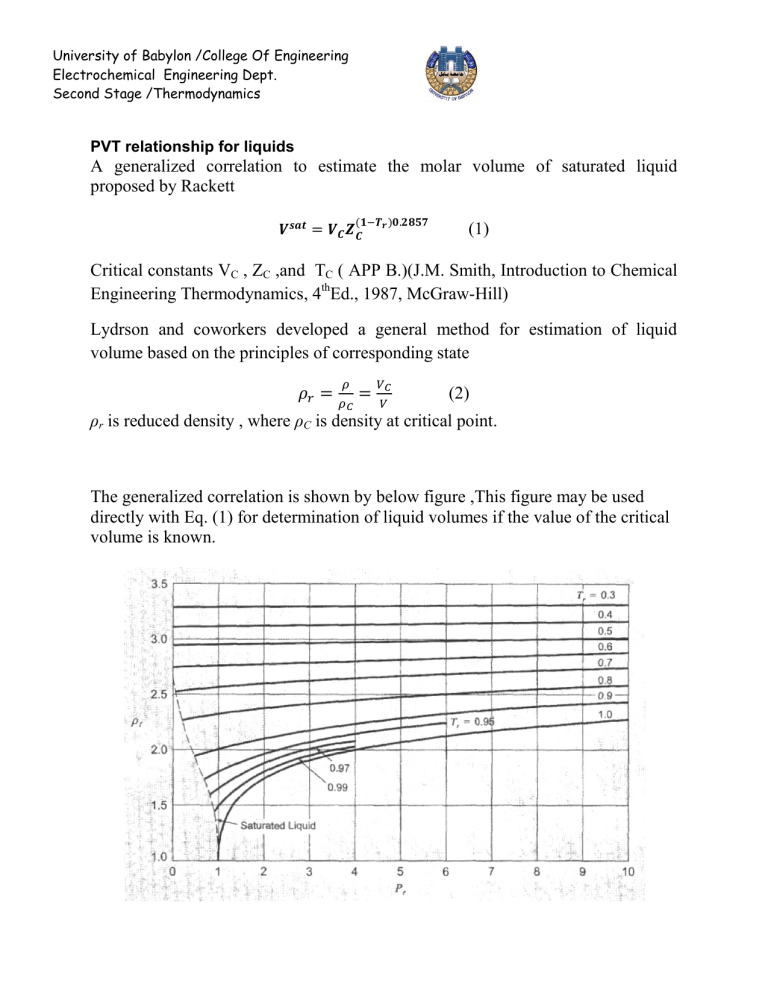
The generalized correlation is shown by below figure ,This figure may be used directly with Eq. (1) for determination of liquid volumes if the value of the critical volume is known.
University of Babylon /College Of Engineering
Electrochemical Engineering Dept.
Second Stage /Thermodynamics
A better procedure is to make use of a single known liquid volume (state 1) by the identity,
V
2
V
1
r r 1
2
V
2
= required volume
V
1
= known volume
r1
,
r2
= reduced densities read from figure