Document 12919600
advertisement

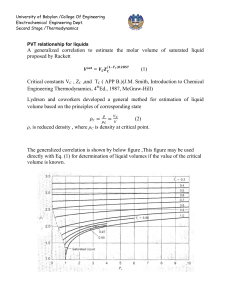
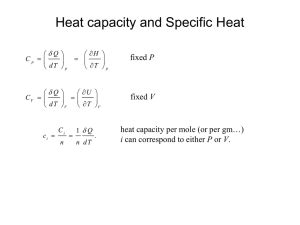
University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Thermodynamic properties of Fluids PROPERTY RELATIONS FOR HOMOGENEOUS PHASES At constant temperature and pressure G represent the part of work may be converted to useful work; G H TS (where G is Gibbs free energy) While A U TS represent from internal energy cab be converted to useful work at constant temperature and volume (where A is Holmholtz free energy) .So we can express for energy H, U, P, V, G and A.Each material has these kinds of energy and it was distributed for any body and sum of all these energies is Enthalpy H H U PV G H TS A U TS U Q W dU dQ dW dU TdS PdV ...............(1) dH dU d ( PV ) dU PdV VdP TdS PdV PdV VdP dH TdS VdP.............(2) dA dU d (TS ) TdS PdV TdS SdT dA PdV SdT .........(3) dG dH d (TS ) TdS VdP TdS SdT dG VdP SdT ..............(4) 1 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics If F=F(x, y) then the total differential of F id define as F F dy dF dx x y y x or Mdx Ndy F x y Where M F and N y x By further differentiation obtain M y 2F x yx 2F N and x y xy U =U(S, V) U U dU dS dV S V V S U U T , and P S V V S H =H(S, P) H H dH dS dP S P P S H H T , and V S P P S A =A (V, T) A A dA dV dT V T T V A A P, and S V T T V G =G (P, T) G G dG dP dT P T T P G G V , and S S T T P 2 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics By apply differentiation on equations 1 through 4, obtain T P ......(5) V S S V T V .......(6) P S S P P S .......(7) T V V T V S ........(8) T P P T These are known as Maxwell`s equations Enthalpy and Entropy as Functions of T and P To know how H and S vary with Temperature and Pressure or in other word we need to know H H S S , , , and T P P T T P P T H C P ......(9) T P H S T .............(10) From equation 2, T P T P C S P .....(11) Combination equation 9 and 10, T T P From heat capacity definition, H S Combination equation 2 and 8 T V P T P T H V V T .....(12) P T T P If H=H (T, P) and, S=S (T, P) dH dH dH dT dP dT P dP T V dH C P dT V T dP....(13) T P dS dS dS dT dP dT P dP T and dS C P and 3 dT V dP....(14) T T P University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics These are general equation relating the enthalpy and entropy of homogenous fluids of constant composition to temperature and pressure. Internal Energy as a Function of P The pressure dependence of internal energy is obtained by differentiation U =H-PV U H V p V P T P T P T The Ideal-Gas State PVig = RT V ig R and use this equation in equation (13) and (14) result : T P P dH ig CPig dT dT R dS ig C Pig dP T P Alternative Forms for Liquids By use volume expansivity (β) definition with equations 8 and 12 result dS ig V .......(15) dP T dH ig (1 T )V .......(16) dP T U (P T )V ............17 P T Where (κ) isothermal compressibility These equations, incorporating β and κ, although general, are usually applied only to liquids. However, for liquids not near the critical point, the volume itself is small, as are β and κ. Thus at most conditions pressure has little effect on the properties of liquids.The important special case of an incompressible fluid. Equation (16) and (17) which required value of β and κ are usually applied only to liquids When (δV /δT) P is replaced in equations 13 and 14 dH CP dT V (1 T )dP.......18 dT dS CP VdP.................19 T 4