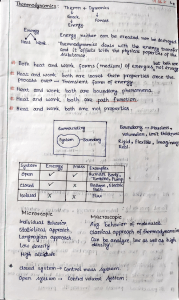
Document
advertisement
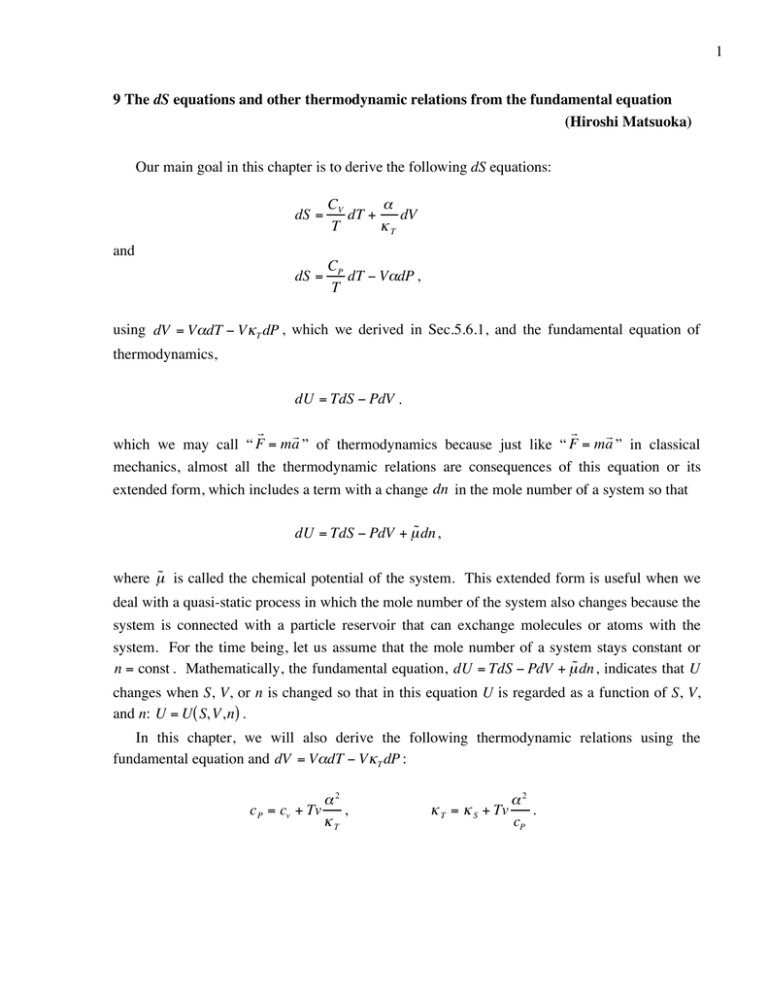
1 9 The dS equations and other thermodynamic relations from the fundamental equation (Hiroshi Matsuoka) Our main goal in this chapter is to derive the following dS equations: dS = CV ! dT + dV T "T dS = CP dT ! V"dP , T and using dV = V!dT " V # T dP , which we derived in Sec.5.6.1, and the fundamental equation of thermodynamics, dU = TdS ! PdV . r r r r which we may call “ F = ma ” of thermodynamics because just like “ F = ma ” in classical mechanics, almost all the thermodynamic relations are consequences of this equation or its extended form, which includes a term with a change dn in the mole number of a system so that dU = TdS ! PdV + µ˜ dn , where µ˜ is called the chemical potential of the system. This extended form is useful when we deal with a quasi-static process in which the mole number of the system also changes because the system is connected with a particle reservoir that can exchange molecules or atoms with the system. For the time being, let us assume that the mole number of a system stays constant or n = const . Mathematically, the fundamental equation, dU = TdS ! PdV + µ˜ dn , indicates that U changes when S, V, or n is changed so that in this equation U is regarded as a function of S, V, and n: U = U( S, V,n) . In this chapter, we will also derive the following thermodynamic relations using the fundamental equation and dV = V!dT " V # T dP : c P = cv + Tv !2 , "T ! T = ! S + Tv "2 . cP 2 9.1 The first dS equation: dS = (CV T )dT + (! " T )dV ( n = const ) In this section, we will derive the first dS equation, dS = CV ! dT + dV , T "T using the fundamental equation as well as a relation, (! P ! T )v = " # T , derived from dv = v !dT " v# T dP in Sec.5.6.1. We start with the following calculus equation for dS: " !S % " !S % dS = $ ' dT + $ ' dV , # !T & V , n # !V & T, n where we regard S as a function of T, V, and n: S = S (T, V,n ) . Note that there is no term with dn as we assume that n is kept constant. In Sec.8.2, we have already shown " !S % C $ ' = V # !T & V , n T so that dS = " !S % CV dT + $ ' dV . # ! V & T ,n T We then need to show " !S % " ! P% " !P % ( $ ' =$ ' = $ ' = , # !V & T ,n # !T & V , n # !T & v ) T where the first equality is one of so-called Maxwell’s relations. The second equality holds because the pressure P is an intensive variable so that it can be regarded as a function of two intensive variables, the temperature T and the molar volume v, so that P = P (T, v ) and because keeping V and n constant in (! P !T )V , n is equivalent to keeping v constant in (! P !T )v . As mentioned above, in Sec.5.6.1, we have already derived the third equality, (! P !T )v = " # T , from dv = v !dT " v# T dP . To show the first equality, we use the Helmholtz free energy introduced and defined, in Sec.8.2, by F ! U " TS . 3 Using the fundamental equation of thermodynamics, dU = TdS ! PdV , we then find dF = d(U ! TS) = dU ! d(TS ) = TdS ! PdV ! (TdS + SdT ) = !SdT ! PdV or dF = !SdT ! PdV , where we have used the following two calculus formulas (see Appendix 5): d ( f ± g) = df ± dg and d ( fg ) = gdf + fdg , where both f and g are functions of two variables x and y. Note that since in this section we assume that the mole number n is kept constant, U and S can be regarded as functions of T and V so that x and y in these calculus formulas correspond to T and V. Comparing dF = !SdT ! PdV with the following calculus equation, " !F % " !F % ' dT + $ ' dV , dF = $ # ! T & V ,n # ! V & T ,n we now find # " F& S = !% ( $ " T ' V ,n and # "F & ( . P = !% $ "V ' T, n This procedure of creating the new variable F from the old variable U is called “the Legendre transformation” and it creates the function F = F (T ,V,n) from the function U = U( S, V,n) by subtracting TS from U so that the old independent variable S is replaced by the new independent variable T. We are now ready to show the above Maxwell’s relation: " ! " ! F% % " ! " !F % % " !S % " ! P% $ ' =$ $( ' ' = $ $( ' ' =$ ' , # !V & T ,n # !V # ! T & V ,n & T, n # !T # ! V & T ,n & V ,n # ! T & V ,n 4 where we have used a calculus formula for a function z = f ( x, y) : ( ! " !f % + ( ! " !f % + * $ ' - =* $ ' - . ) ! x # !y & x , y ) !y # !x & y , x There are many other Maxwell’s relations besides the one we have just derived: " !S % " ! P% $ ' =$ ' . # !V & T ,n # !T & V , n Many of these relations connect a partial derivative whose physical meaning is obscure (e.g., the LHS of the above equation) with another partial derivative whose physical meaning is relatively clear (e.g., the RHS of the above equation). 9.2 The second dS equation, dS = (CP T) dT ! V"dP ( n = const ) and c P = cv + Tv! 2 " T In this section, we will derive the second dS equation, dS = CP dT ! V"dP , T using the first dS equation and dv = v !dT " v# T dP . We start with the following calculus equation for dS: " !S % " !S% dS = $ ' dT + $ ' dP , # !T & P ,n # !P & T, n where we regard S as a function of T, P, and n: S = S (T, P, n ) . Note that there is no term with dn as we assume that n is kept constant. In Sec.8.1, we have already shown " !S % C $ ' = P # !T & P,n T so that dS = " !S % CP dT + $ ' dP . # !P & T ,n T Using the first dS equation and dV = V!dT " V # T dP derived in Sec.5.6.1, we also find 5 dS = 2' $ CV ! C ! (V!dT # V "T dP ) = 1 & CV + TV ! ) dT # V!dP . dT + dV = V dT + T "T T "T T% "T ( Comparing these two equations for dS in terms of dT and dP, we then arrive at the second dS equation, dS = CP dT ! V"dP , T as well as CP = CV + TV !2 , "T from which we obtain c P = cv + Tv !2 , "T which we have used to calculate cv from c P . We also find " !S % " !V % $ ' = (V ) = ( $ ' , # !P & T ,n # !T &P which is another example of Maxwell’s relations and from which we obtain " !v % " !s % $ ' = ($ ' , # !T & P # !P & T which we have used in showing ! " 0 as T ! 0 : 9.3 ! T = ! S + Tv" 2 cP from dV = V!dT " V # T dP and the second dS equation In this sub-section, we will derive ! T = ! S + Tv "2 , cP 6 which we have used to calculate ! T from ! S in Sec.5.6.5. compressibility ! S is defined by !S " # Recall that the adiabatic 1 % $V ( ' * . V & $ P ) No heat transfer, n Since the entropy of a system remains constant during an infinitesimal quasi-static adiabatic process, we can express ! S as !S = " 1 $ #V ' & ) , V % # P ( S,n which suggests that we should regard the volume as a function of the entropy and the pressure of the system so that " !V % " !V % " !V % dV = $ ' dS + $ ' dP = $ ' dS ( V) S dP . # ! S & P ,n # ! P & S,n # ! S & P ,n If we eliminate dT in dV = V!dT " V # T dP using the second dS equation, dS = CP dT ! V"dP , T then we can express ! S in terms of ! , ! T , and CP . By solving the second dS equation for dT, we obtain dT = T TV! dS + dP CP CP so that dV = V!dT " V # T dP = $ TV! ! 2 ') dS " V && # T " TV dP . CP CP )( % and ! S = ! T " TV #2 #2 = ! T " Tv , CP cP ! T = ! S + Tv "2 . cP which is equivalent to 7 SUMMARY FOR CH.9 1. The Helmholtz free energy F of a system defined by F ! U " TS satisfies dF = !SdT ! PdV , which follows from the fundamental equation of thermodynamics, dU = TdS ! PdV , and implies # " F& S = !% ( and $ " T ' V ,n # "F & P = !% ( , $ "V ' T, n which leads to " ! " ! F% % " ! " !F % % " !S % " ! P% ) . $ ' = $$ $ ( ' '' = $$ $( ' '' = $ ' = # !V & T ,n # !V # ! T & V ,n & T, n # !T # ! V & T ,n & V ,n # ! T & V ,n * T so that " !S % " !S % C ( dS = $ ' dT + $ ' dV = V dT + dV , # !T & V , n # !V & T, n T )T (the first dS equation) where we have also used the following relation derived in Sec.8.1: " !S % C $ ' = V. # !T & V , n T 2. The first dS equation and dV = V!dT " V # T dP lead to dS = 1 #% ! 2 &( C C + TV dT ) V!dP = P dT ) V!dP . (the second dS equation) % V ( T$ "T ' T from which we obtain CP = CV + TV !2 "T or c P = cv + Tv !2 . "T 3. The second dS equation together with dV = V!dT " V # T dP leads to ! T = ! S + Tv "2 . cP