considered. Various items for this system per unit of mass... 10-104
advertisement
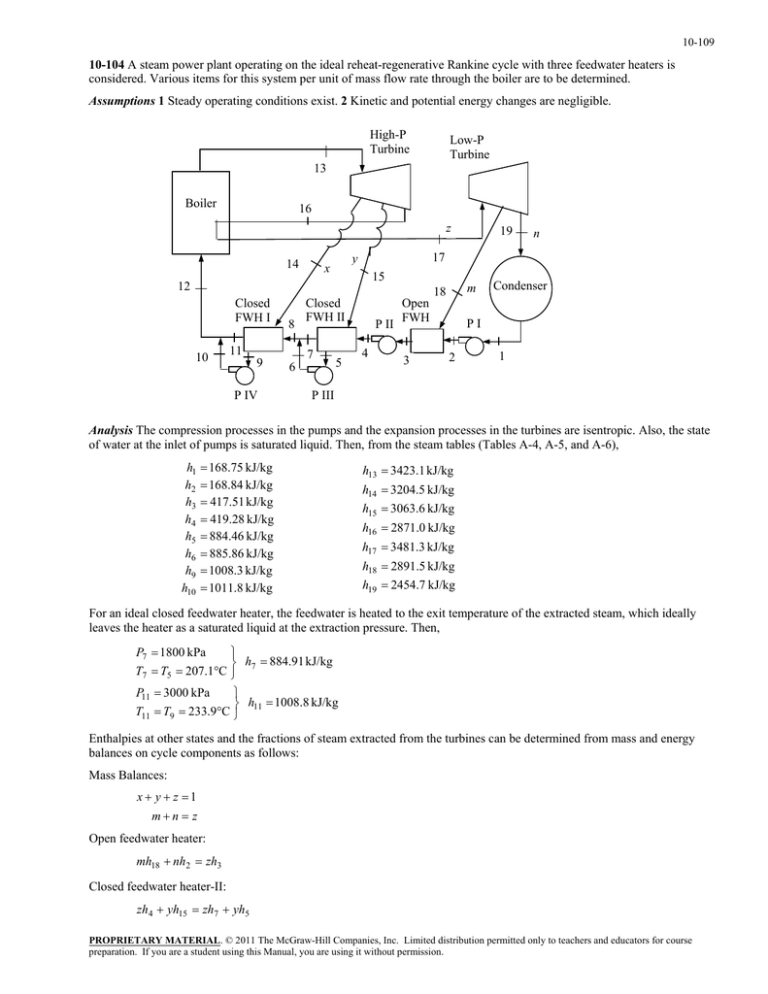
10-109 10-104 A steam power plant operating on the ideal reheat-regenerative Rankine cycle with three feedwater heaters is considered. Various items for this system per unit of mass flow rate through the boiler are to be determined. Assumptions 1 Steady operating conditions exist. 2 Kinetic and potential energy changes are negligible. High-P Turbine Low-P Turbine 13 Boiler 16 z 14 15 12 Closed FWH I 10 8 11 9 P IV Closed FWH II 7 6 5 n 17 y x 19 P II 4 Open FWH 3 m 18 Condenser PI 2 1 P III Analysis The compression processes in the pumps and the expansion processes in the turbines are isentropic. Also, the state of water at the inlet of pumps is saturated liquid. Then, from the steam tables (Tables A-4, A-5, and A-6), h1 h2 h3 h4 h5 h6 h9 h10 168.75 kJ/kg 168.84 kJ/kg 417.51 kJ/kg 419.28 kJ/kg 884.46 kJ/kg 885.86 kJ/kg 1008.3 kJ/kg 1011.8 kJ/kg h13 3423.1 kJ/kg h14 3204.5 kJ/kg h15 3063.6 kJ/kg h16 2871.0 kJ/kg h17 3481.3 kJ/kg h18 2891.5 kJ/kg h19 2454.7 kJ/kg For an ideal closed feedwater heater, the feedwater is heated to the exit temperature of the extracted steam, which ideally leaves the heater as a saturated liquid at the extraction pressure. Then, P7 1800 kPa h7 884.91 kJ/kg T7 T5 207.1C P11 3000 kPa h 1008.8 kJ/kg T11 T9 233.9C 11 Enthalpies at other states and the fractions of steam extracted from the turbines can be determined from mass and energy balances on cycle components as follows: Mass Balances: x y z 1 mn z Open feedwater heater: mh18 nh2 zh3 Closed feedwater heater-II: zh4 yh15 zh7 yh5 PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission. 10-110 Closed feedwater heater-I: ( y z )h8 xh14 ( y z )h11 xh9 Mixing chamber after closed feedwater heater II: zh7 yh6 ( y z )h8 Mixing chamber after closed feedwater heater I: xh10 ( y z )h11 1h12 Substituting the values and solving the above equations simultaneously using EES, we obtain h8 885.08 kJ/kg h12 1009.0 kJ/kg x 0.05334 y 0.1667 z 0.78000 m 0.07124 n 0.70882 Note that these values may also be obtained by a hand solution by using the equations above with some rearrangements and substitutions. Other results of the cycle are wT,out,HP x(h13 h14 ) y (h13 h15 ) z (h13 h16 ) 502.3 kJ/kg wT,out,LP m(h17 h18 ) n(h17 h19 ) 769.6 kJ/kg q in h13 h12 z (h17 h16 ) 2890 kJ/kg q out n(h19 h1 ) 1620 kJ/kg th 1 q out 1620 1 0.4394 43.9% q in 2890 PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.










