Chemistry 114 First Hour Exam Name:____________ All problems are worth 12 points
advertisement

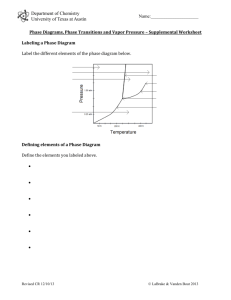
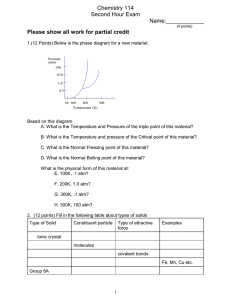
Chemistry 114 First Hour Exam Name:____________ (4 points) Please show all work for partial credit All problems are worth 12 points 1. Define the following terms: Viscosity- Resistance to flow Adhesive forces - Forces that occur at the interface between two substances. Normal boiling point - The temperature at which a liquid has a vapor pressure of 1 atm. Azeotrope - A solution that boils with a constant composition, so its components cannot be purified by distillation. Critical temperature - The temperature above which a gas cannot be condensed to its liquid form, no matter how high the pressure. First order reaction - A reaction with an order parameter of 1. 2. When I shine an X-ray with a wavelength of 1.54Å at a crystal, I get a reflection from the crystal at 38.4o. What is the distance between atom layers in this crystal. (Assume that n=1) 1 3. Below are 4 pairs of solids. melting point and why. For each pair circle the compound with the HIGHER NaF and CH3F Why? CH3F and CF4 Why? Ionic compound Charge-Charge forces found in Ionic compound are very strong CH3F is polar which gives it a stronger interactive force than the non-polar CF4 CF4 and CBr4 Why? CH3OH and CH2OHCH2OH Why? Both compounds are nonpolar and held together by London forces But London forces are proportional To the number of electrons so CBr4 has more electrons and a stronger London force The OH in both compounds has a potential to make strong hydrogen bonds, but CH2OHCH2OH has 2 OH’s so it can make twice as many hydrogen bonds 4. I am going to make a solution by mixing 10 grams of NaOH with 90 grams of water A. What is the weight or mass % of NaOH in this solution? B. What is the mole fraction of NaOH in this solution C. Assuming the solution has a volume of 95 mls, what is the molarity of NaOH in the solution? D. What is the colligative molality of the solution? 2 5. I am going to make a solution with 50 grams of methanol (CH3OH) and 50 grams of ethanol (CH3CH2OH). What is the mole fraction of each liquid in this solution? If the vapor pressure of methanol is 260 torr @ 40o C, and ethanol has a vapor pressure of 150 torr at 40oC, what is the vapor pressure of this solution at 40oC? What is the mole fraction of methanol in this vapor? If the actual vapor pressure of the solution was LOWER than your answer in part B, would that be a positive or negative deviation from Raoult’s law, and what would that indicate about the interactions between methanol and ethanol in solution? When the actual vapor pressure is lower than predicted by Raoult’s law this is a negative deviation from Raoult’s law and this indicates strong interactions between the solvent and solute. 6.You may have heard of the ‘Bends’ a painful, potentially fatal problem that occurs when divers ascend too fast. It is caused by air bubbles forming in the bloodstream as a diver comes up from the bottom and nitrogen dissolved in their blood forms bubbles in the bloodstream. A. If the Henry’s Law constant for N2 is 1600 atm/M, and we assume that at sea level the pressure of N2 is 0.8 atm, what is the molar concentration of N2 in your blood. B. If you dive to 66 feet, where the pressure on your body is 3 atm, what is the molar concentration of N2 in your blood now? C. If an average body contains 5.5 liters of blood, use the difference between your answers for A and B to determine the volume of bubbles in your blood that could form as a diver rises from 66 feet to the surface assuming the temperature is 25oC. The Ä molarity = .00188-.0005 or .00138M .00138 moles/Liter X 5.5 liters = .00756 moles of N2 must escape from the blood using good ol’ PV=nRT V=nRT/P V = (.00756 molesA.08206 lAAtm/KAmol A298K)/1atm =.186L or 186 mLs of gas that needs to escape from the blood 3 7. I am investigating the reaction A + B 6C. obtained the data shown in the table below: [A](M) [B] (M) 0.7 1 1 Using the method of initial rates I have rate M/min 0.7 0.7 1.4 1.37 1.82 3.64 What are the order parameters for A and B in this reaction, and what is the rate constant for this reaction? 8. I have a first order reaction with a rate constant of .1 min-1. If the initial concentration of my reactant, Z is 1.5 M, how long will it take for the concentration of Z to reach .75M? Two ways to do this. 1. If you notice that the second concentration is ½ of this first, you can do this with the half-life for a first order reaction t1/2 = .693/k = .693/.1 min-1 = 6.93 minutes 2. If you don’t see that this is a half-life problem, then you must use the equation ln[A]t = ln[A]0 -kt ln(.75)=ln(1.5)-.1X -.288=.405 -.1X -.693=-.1X .693=.1X X=.693/.1 =6.93 min 4