Protein folding in the cell (I)
advertisement
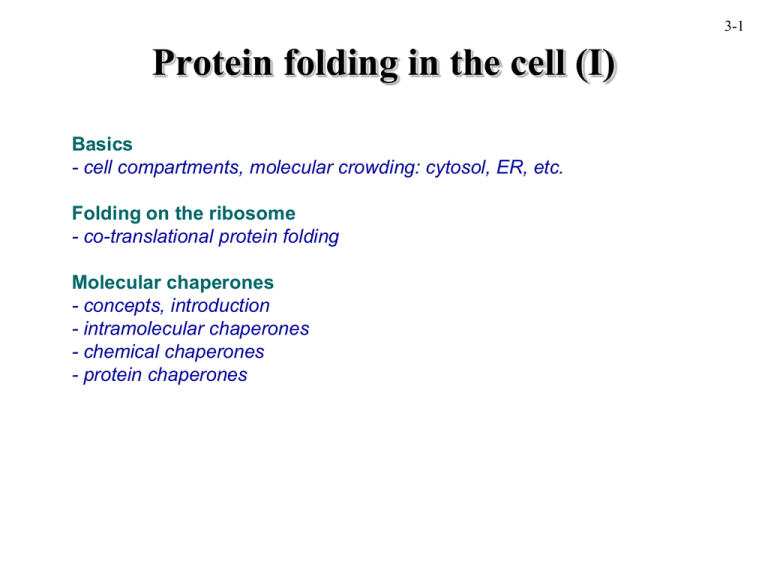
3-1 Protein folding in the cell (I) Basics - cell compartments, molecular crowding: cytosol, ER, etc. Folding on the ribosome - co-translational protein folding Molecular chaperones - concepts, introduction - intramolecular chaperones - chemical chaperones - protein chaperones Cell compartments and folding 3-2 • eukaryotes - cytosol ..................................protein synthesis, folding/assembly - extracellular .........................proteins are exported in folded form - mitochondria ........................limited protein synthesis; energy production - chloroplasts ..........................limited protein synthesis; light harvesting - endoplasmic reticulum.......... import of unfolded proteins; protein processing - peroxisome ........................... import of folded proteins; anab./catab. pathways - nucleus ................................. import of folded proteins - lysosome................................import of unfolded proteins; degradation • bacteria - cytosol ..................................protein synthesis, etc. - periplasm .............................import and folding of periplasmic proteins - extracellular .........................proteins are exported • archaea - cytosol ..................................protein synthesis, etc. - extracellular .........................proteins are exported 3-3 Folding in vitro vs. in vivo in vitro in vivo protein denatured in a chaotrope Differences: folding by dilution in buffer folded protein 1. One has all of the information immediately available for folding; the other process is gradual 2. the cellular environment is very different (much more crowded) folding folded protein 3-4 Co-translational protein folding Fact: - first ~30 amino acids of the polypeptide chain present within the ribosome is constrained (the N-terminus emerges first) folding assembly Assumption: as soon as the nascent chain is extruded, it will start to fold co-translationally (i.e., acquire secondary structures, super-secondary structures, domains) until the complete polypeptide is produced and extruded 3-5 Sindbis Virus Capsid Protein (SCP) • SCP is the capsid protein of the Sindbis virus • 26S Sindbis RNA encodes a polyprotein N C SCP E1 E2 E3 • SCP is auto-proteotically cleaved from the rest of the polyprotein • other cellular proteases cleave E1-E3 from the polyprotein to generate the mature proteins; E1, the envelope protein, is 9 kDa • SCP is a 33 kDa serine protease • WT SCP self-cleaves; Ser215 => Ala215 mutant doesn’t catalytic triad & C-terminus of SCP 3-6 SCP folds co-translationally Experiment: 1. make and translate different SCP construct RNAs in vitro in the presence of 35S-methionine for 2 min 2. Prevent re-initiation of translation with aurintricarboxylic acid (ATCA): ‘synchronizing’ 3. at set timepoints, add SDS buffer and perform SDS-PAGE 4. observe by autoradiography 2 Result: * N C SCP E1 C SCP C SCP E1 5 6 7 8 10 12 min 42 kDa 33 kDa 9 kDa 3 4 5 6 7 8 10 12 min 42 kDa 33 kDa 9 kDa WT SCP 2 N 4 Mut SCPE1 2 N 3 WT SCPE1 3 4 5 6 7 8 10 12 min 42 kDa 33 kDa 9 kDa Macromolecular crowding in vitro E. coli cytosol ~340 mg/ml <0.1 mg/ml Ellis and Hartl (1996) FASEB J. 10:20-26 ribosome proteins chaperonin nucleic acids other macromolecules When doing experiments in vitro, we should all be thinking about this: proteins in isolated (pure) systems may not behave as they do in the cell - binding partner(s) might be missing - post-translational modifications might be missing - cell conditions (pH, salts, etc. may be dramatically different 3-7 3-8 Effects of crowding Definition: Molecular crowding is a generic term for the condition where a significant volume of a solution, or cytoplasm for example, is occupied with things other than water Fact: - association constants (ka) increase significantly - dissociation constants (kd) decrease significantly (kd=1/ka) - increased on-rates for protein-protein interactions (see for example Rohwer et al. (2000) J. Biol. Chem. 275, 34909) Assumption: - non-native polypeptides will have greater tendency to associate intermolecularly, enhancing the propensity of aggregation 3-9 Effects of crowding: example denatured lysozyme, reduced or oxidized dilution in buffer with different crowding agents measure lysozyme activity oxidized lysozyme loss of activity due to protein aggregation reduced lysozyme crowding agents: ficoll 70*, dextran 70, protein (BSA, ovalbumin) *roughly spherical polysaccharide van den Berg et al. (1999) EMBO J. 18, 6927. 3-10 Problem: non-native proteins • non-native proteins expose hydrophobic residues that are normally buried within the ‘core’ of the protein • these hydrophobic amino acids have a strong tendency to interact with other hydrophobic (apolar) residues - especially under crowding conditions exposed hydrophobic residues X X X intramolecular incorrect molecular interactions & loss of activity X intermolecular X X X misfolding X X X aggregation 3-11 Solution: molecular chaperones • in the late 1970’s, the term molecular chaperone was coined to describe the properties of nucleoplasmin: Nucleoplasmin prevents incorrect interactions between histones and DNA Laskey, RA, Honda, BM, Mills, AD, and Finch, JT (1978). Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature 275, 416-420. Dictionary definition: 1: a person (as a matron) who for propriety accompanies one or more young unmarried women in public or in mixed company 2: an older person who accompanies young people at a social gathering to ensure proper behavior; broadly : one delegated to ensure proper behavior • in the late 1980’s, the term molecular chaperone was used more broadly by John Ellis to describe the roles of various cellular proteins in protein folding and assembly Molecular chaperones: general concepts Requirements for a protein to be considered a chaperone: (1) interacts with and stabilizes non-native forms of protein(s) - technically also: folded forms that adopt different protein conformations (2) not part of the final assembly of protein(s) Functions of a chaperone: self-assembly refers to the folding of the polypeptide, as well as to its assembly into functional homo- or hetero-oligomeric structures “classical” - assist folding and assembly assisted self-assembly more recent - modulation of conformation (as opposed to spontaneous self-assembly) - transport - disaggregation of protein aggregates - unfolding of proteins prevention of assembly assisted disassembly 3-12 Molecular chaperones: common functional assays Type of assay Rationale Binary complex formation If chaperone has high enough affinity for an unfolded polypeptide, it will form a complex detectable by: • co-migration by SEC; • co-migration by native gel electrophoresis • co-immunoprecipitation Prevention of aggregation Binding of chaperones to non-native proteins often reduces or eliminates their tendency to aggregate. Assay may detect weaker interactions than is possible with SEC Refolding Chaperones stabilize non-native proteins; some can assist the refolding of the proteins to their native state. Usually, chaperones that assist refolding are ATP-dependent Assembly Some chaperones assist protein complex assembly Activity Some chaperones modulate the conformation/activity of proteins (Miscellaneous) A number of chaperones have specialized functions 3-13 3-14 Intramolecular chaperones Concept: - portions of a polypeptide may assist the biogenesis of the mature protein without being part of the final folded structure - these regions are chaperones by definition, although “classical” molecular chaperones act inter-molecularly, not intra-molecularly. Intramolecular chaperone: example 3-15 acid-unfolded; with 77aa propeptide Subtilisin E - non-specific protease - mature protein cannot fold properly if propeptide is removed Gdn-HCl unfolded; with propeptide Gdn-HCl unfolded; without 77aa propeptide propeptide (77 aa) precursor (352 aa) mature protein (275 aa) Shinde et al. (1993) PNAS 90, 6924. Intramolecular chaperone: continued Subtilisin E propeptide - unstructured alone in solution - alpha-helical when complexed with subtilisin? propeptide is ~ 20% of preprotein; CD suggests combination mature subtilisin + propeptide mostly helical alpha-helical: minima @ 208, 222 nm maximum @ 192 nm - more pronounced minimum at 208 nm compared to 222 nm suggests less helical Structure propeptide in TFE ellipticity Interpretation of CD data propeptide with subtilisin alpha propeptide beta coil subtilisin nm beta-sheet: minimum @ 220 nm Maximum @ 193 nm random coil: maximum ~220 nm Note:CD traces are additive Propeptide must interact with subtilisin 3-16 Intramolecular cleavage or intermolecular? Result: 3-17 released propeptide Fact: unfolded His10-preprotein can refold alone in solution Experiment: 1. prepare subtilisin pre-protein containing an N-terminal polyhistidine tag (His10) 2. unfold in denaturant 3. bind different concentrations of the protein to Ni2+-NTA resin 4. assay for folding by measuring propeptide release Q: what do the results mean? Q: why bind the protein to a resin? Q: why use different concentrations of proteins? full-length protein Li et al. (1996) J. Mol. Biol. 262, 591. 3-18 Chemical chaperones Concept: - small molecules could enhance the stability and assist the folding or assembly of proteins - under conditions of cellular stress, such as a heat-shock, small molecules may help proteins from misfolding and aggregating - one easy way to test is to see how they can prevent loss of activity, or, prevent the aggregation of a protein - protein aggregation can be conveniently monitored spectrophotometrically at 360 nm, where light scattering from the aggregates is detected 3-19 A Firefly-luc F-luc in GuHCl F-luc in GuHCl B protein aggregation in vitro studies Chemical chaperones: example Singer and Lindquist (1998) Mol. Cell 1, 639. Chemical chaperones: example bacterial luciferase expressed in yeast; subjected to heat shock conditions in vivo studies B C 40ºC heat shock tps1 yeast cells have a deletion in the trehalose synthase 40ºC heat shock 3-20 without with protein aggregation protein aggregation Different chemical chaperones glycerol is often used to stabilize proteins in vitro 3-21 trans-acting protein molecular chaperones - cis-acting (intramolecular) chaperones are relatively rare - chemical chaperones may play an important role in protecting proteins in the cell, but their extent of action is likely to be limited - organisms have evolved large families of protein molecular chaperones that have either general functions in the cell, or have highly specific functions - the expression of many of the chaperones is induced under cellular stress conditions--giving rise to the name “Heat-shock proteins”, or Hsps, followed by their Molecular Weight (MW) BUT: - not all chaperones are Hsps - not all Hsps are chaperones Best characterized: small Hsps (12-42 kDa), Hsp40, Hsp60 (chaperonins), Hsp70, Hsp90, Hsp100/Clp/AAA ATPases 3-22 3-23 Functional proteins from a random-sequence library Anthony D. Keefe & Jack W. Szostak Nature 410, 715-718 (2001) The PDF file of this manuscript is available on the MBB443 web site There will be one question on the first exam relating to this paper