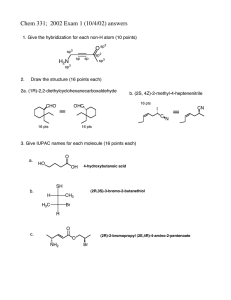
Chem 331: Problem Set #4 (Hour Test practice questions) O (2S, 4Z)-4-bromo-2-methyl-4-hexenal Br
advertisement

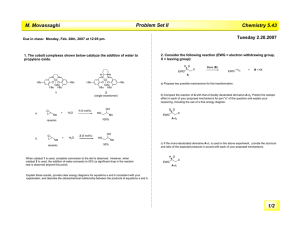
Chem 331: Problem Set #4 (Hour Test practice questions) 1) Draw the structure: (2S, 4Z)-4-bromo-2-methyl-4-hexenal O Br 2) Give the IUPAC name for each compound Br O O CHO (2S)-2-bromopropyl (3S)-3-methylpentanoate (1S,3R)-3-methylcyclohexane carboxaldehyde OH CH3 Cl H H Br HS Br H CH3 H (1R,4S,5R)-4-bromo-5-mercapto-2-cycloheptenol (2R,4S)-2-bromo-4-chloropentane 3) For each pair, indicate which is more stable. Use a clear picture to explain why Both cyclohexanes are 'locked' with equatorial tBu's and axial methyl groups so the answer lies in the double bond Br Br t-Bu Br Br Brax H H Breq Breq Breq H Brax H H H CH3 H Brax H Brax H CH3 H tBu t-Bu most stable form has no axial Br's Breq t-Bu H H sp 2 center There is no axial group here, and therefore there is only one 1,3-diaxial interaction make a model to convince yourself! two 1,3-diaxial interactions Chem 331: Problem Set #4 (Hour Test practice questions) 4) 1,2-cycloheptadiene is not a stable molecule. Use a clear picture and less than 20 words to explain why. Begin by drawing the MO for the allene (C=C=C) fragment C C C the central carbon is sp hybridized, and the ideal geometry for this fragment is linear C C C C that puts these two carbons far away from one another, and you only have 2 carbons left to form the 7-membered ring. It is impossible to do it without severly bending the allene away from its ideal geometry of 180 ° (approximately a 30 ° distortion is required) BUILD A MODEL! 5) Provide a mechanism Br Bu3SnH O O H O AIBN H NC N N • H CN Br SnBu3 multiple bond addition Br-atom extraction • O O O H-atom extraction O Bu3Sn • SnBu3 H CN AIBN • SnBu3 :N N: + O H H HH O H HH H-abstraction H • H O H + O • O O multiple bond addition Having trouble getting started? Map it out first. Start with the acetal as your marker. You can see that it is one carbon away from a 5 membered ring Br O 1 3 2 O 3 O 1 2 O Now, we can see that the central 5-membered ring of the product is the ring from the starting material 6 O 7 1 2 3 5 6 Br 7 O O 3 5 4 1 O 2 4 finish labeling. We can see that a bond must be formed between the atoms indicated below Br e c 6 b a d O 7 3 5 4 1 2 O e 6 7 c b 3 5 a 4 2 d O O O