Chem 331; 2002 Exam 1 (10/4/02) answers O H N
advertisement
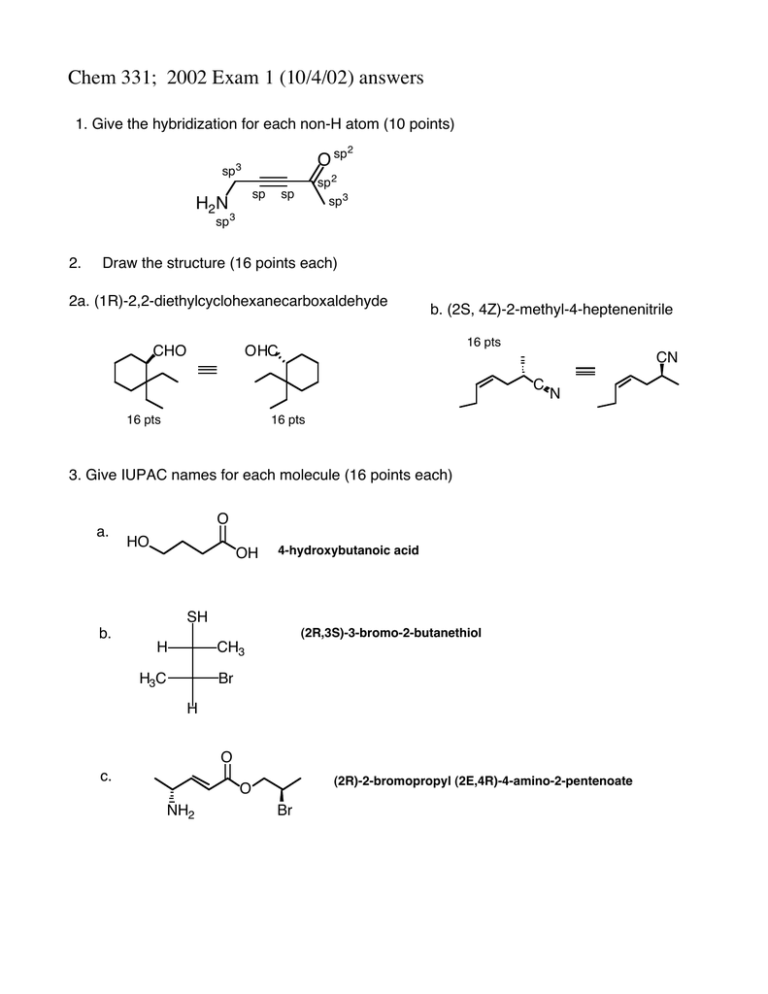
Chem 331; 2002 Exam 1 (10/4/02) answers 1. Give the hybridization for each non-H atom (10 points) O sp sp 3 sp H2 N 2 sp 2 sp 3 sp sp 3 2. Draw the structure (16 points each) 2a. (1R)-2,2-diethylcyclohexanecarboxaldehyde CHO b. (2S, 4Z)-2-methyl-4-heptenenitrile 16 pts OHC CN C 16 pts N 16 pts 3. Give IUPAC names for each molecule (16 points each) a. O HO OH 4-hydroxybutanoic acid SH b. (2R,3S)-3-bromo-2-butanethiol H CH3 H3C Br H O c. (2R)-2-bromopropyl (2E,4R)-4-amino-2-pentenoate O NH2 Br Chem 331; 2002 Exam 1 (10/4/02) answers 4. (20 points each) For each pair of cyclohexanes, which is more stable. Explain your reasoning in detail (no credit for a correct guess, only a correct explanation) a Etequatorial tBu tBu Et H A tBu H Etaxial H B more stable H tBu Et For both A and B, the favored conformation has the t-Bu groups in the equatorial positions. This places the Et group of A in the equatorial position, and the Et group of B in the axial position. b Braxial Meaxial Me Br H A Me Brequatorial tBu tBu Br Meequatorial tBu H tBu B more stable For both A and B, the favored conformation has the t-Bu groups in the equatorial positions. A has an axial methyl group, which 'costs' 1.70 kcal (Table 4-3 on pg 142 in V&S). B has an axial bromine, which costs 0.55 kcal/mol. B is more stable. 6. The 'bridgehead' alkene 1 is extremely unstable. Use a clear orbital picture and less than 15 words to explain why. Hint: the answer has to do with the π-bond. 1 side view The σ framework holds the p orbitals at a ~90 °. They cannot overlap and therefore cannot bond. NAME 5. Provide a detailed arrow pushing mechanism for the following reaction Br Bu3 SnH H AIBN O H H O Initiation NC N N CN AIBN Br :N N: + • CN H SnBu3 H-atom extraction • SnBu3 • Br-atom extraction O • O multiple bond addition H O Bu3Sn• H H Bu3Sn H H • O + • SnBu3 H-atom extraction O H H H multiple bond addition NAME 6. The 'bridgehead' alkene 1 is extremely unstable. Use a clear orbital picture and less than 15 words to explain why. Hint: the answer has to do with the π-bond. 1 side view The σ framework holds the p orbitals at a ~90 °. They cannot overlap and therefore cannot bond.