lecture 5 - kinetic theory
advertisement

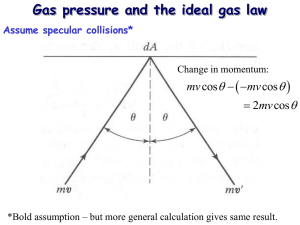
Announcements 9/10/10 Be sure to register your clicker on the class website! (About 5 of you are not registered yet.) a. After everyone is registered, I’ll tell the computer to go back and re-grade all of the earlier quizzes to give you credit. Results of the TA office hour survey, effective next week: a. Mon 3-4 pm b. Wed 5-6 pm c. Fri 5-6 pm My own office hours are still right after class, 23 pm. Video Video: Barrel Crush Worked problems: How much mass does the air in this room have? (MM 0.029 kg/mol) According to the ideal gas law, what is the density of air at 300K? At arbitrary T? A hot air balloon is 520 kg (including passengers). It’s spherical, with radius = 8 m. The temperature is 300K outside (80.3F). How hot does the pilot have to get the air inside the balloon for it to lift off? Thought quiz (ungraded): In air, the molecular mass of oxygen molecules is 32 g/mol; the molecular mass of nitrogen molecules is 28 g/mol. Which molecules are traveling faster on average? a. Oxygen b. Nitrogen c. Same speed Demo: heavy vs light molecules Equipartition Theorem “The total kinetic energy of a system is shared equally among all of its independent parts, on the average, once the system has reached thermal equilibrium.” “independent”: e.g. x, y, z (for translational KE) “parts”: translational, rotational, vibrational Specifically, each “degree of freedom”, of each molecule, has “thermal energy” of … ½kBT Thought quiz Compare a monatomic molecule such as Ne to a diatomic molecule such as O2. If they are at the same temperature(*), which has more kinetic energy? a. Ne b. O2 c. Same d. Not enough information to tell (*) let’s assume the temperature is “high”. Relative to what, we’ll discuss in a minute. Disclaimer Thermal energy (measured by kBT) must be comparable to the quantum energy levels, or some degrees of freedom get “frozen out” From section 21.4: diatomic hydrogen Y-axis: heat added, divided by temperature change (per mole) Units: J/molK Translational KE and vrms Worked problem: what is average speed (vrms) of oxygen molecules at 300K? Molecular View of Pressure Related problem: pressure by baseballs (m = 145 g) on a wall (A = 9 m2). Speed = 85 mph (38 m/s). Elastic collisions, each lasting for 0.05 seconds. (This is the time the ball is in contact with the wall.) A baseball hits the wall every 0.5 seconds. Actual problem: a cube filled with gas a. Pressure on right wall from one molecule b. Pressure on right wall from all molecules Molecular View of Pressure, cont. Result: P = N m ⅓ v2 / V What does PV equal? Compare to: PV = N kB T What does T equal? Quick writing: What does this tell you about what temperature really is? Demo Demo: kinetic theory machine