lecture 5 - kinetic theory

Announcements 9/7/12
Prayer
Lab 1 due tomorrow—turn it in the same place you’ve been turning in the homework
Lab 2 starts tomorrow
Dilbert:
From warmup
Extra time on?
a. Root Mean Square (several people) b. Going over Wednesday's cup problem would be really really good.
Other comments?
(By the way, if I don’t list your comment, please don’t take it personally.)
Worked problems:
How much mass does the air in this room have?
(MM 0.029 kg/mol)
According to the ideal gas law, what is the density of air at 1 atm, for 300 K? For arbitrary
T?
A hot air balloon is 520 kg (including passengers). It’s spherical, with radius = 8 m.
The temperature is 300K outside (80.3
F), pressure is 1 atm. How hot does the pilot have to get the air inside the balloon for it to lift off?
Some answers: 1.175 kg/m 3 ; 378K (221
F)
Clicker question:
In air, the molecular mass of oxygen molecules is 32 g/mol; the molecular mass of nitrogen molecules is 28 g/mol.
Which molecules are traveling faster on average? a. Oxygen b. Nitrogen c. Same speed
Demo: heavy vs light molecules
From warmup
What is it that causes a gas to have a certain temperature? a. Temperature is a measure of the average kinetic energy of the individual molecules.
Mass and velocity.
Demo: heavy vs light molecules
Equipartition Theorem
“The total kinetic energy of a system is shared equally among all of its independent parts, on the average, once the system has reached thermal equilibrium.”
“independent”: e.g. x, y, z (for translational KE)
“parts”: translational, rotational, vibrational
Specifically, each “degree of freedom”, of each molecule, has “thermal energy” of …
½k
B
T
Clicker question:
Compare a monatomic molecule such as
Ne to a diatomic molecule such as O
2
. If they are at the same temperature(*), which has more kinetic energy?
a. Ne b. O
2 c. Same d. Not enough information to tell
(*) let’s assume the temperature is “high”.
Relative to what, we’ll discuss in a minute.
Disclaimer
Thermal energy (measured by k
B
T) must be comparable to the quantum energy levels, or some degrees of freedom get “frozen out”
From section 21.4: diatomic hydrogen
Y-axis: heat added, divided by temperature change (per mole)
Units: J/mol
K
Translational KE and v
rms
Worked problem: what is average speed (v
300K?
rms
) of oxygen molecules at
From warmup
Compare v rms for various gases given in
Table 21.1 to the speed of sound given in Table 17.1. Is there a connection?
Why/why not? a. The higher v rms the faster the speed of sound. Because the molecules are moving faster so it is easier for sound waves to pass through the media.
From warmup
In the molecular model of ideal gases, what is it that causes a gas to exert pressure on its surroundings? a. Collisions against a wall. A lot of little things banging against something gives a seemingly invisible force.
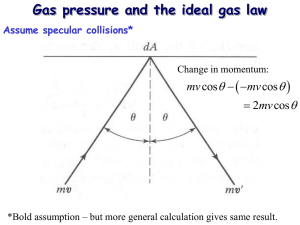
Molecular View of Pressure
Related problem: What is average pressure by baseballs (m = 145 g) on a wall (A = 9 m 2 ).
Speed = 85 mph (38 m/s). Elastic collisions, each lasting for 0.05 seconds. (This is the time the ball is in contact with the wall.) A baseball hits the wall every 0.5 seconds.
Answer: 2.45 Pa
Actual problem: a cube filled with gas a. Pressure on right wall from one molecule?
Answer: 2mv x
/(L 2 t between hits
) = mv x
2 /L 3 b. Pressure on right wall from all molecules
Answer: P = Nmv x
2 /V
Molecular View of Pressure, cont.
Result for v instead of v x
:
P = N m ⅓ v 2 / V
What does PV equal?
Compare to: PV = N k
B
T
What does v equal? What does T equal?
What is temperature? (revisited)
Demo
Demo: kinetic theory machine
Clicker question: Which “molecules” have the most kinetic energy?
a. The heavy ones b. The light ones c. Same
(Repeat) Which ones have the fastest average velocity?