Carboxylic Acids Structure • The functional group of a carboxylic acid is
advertisement
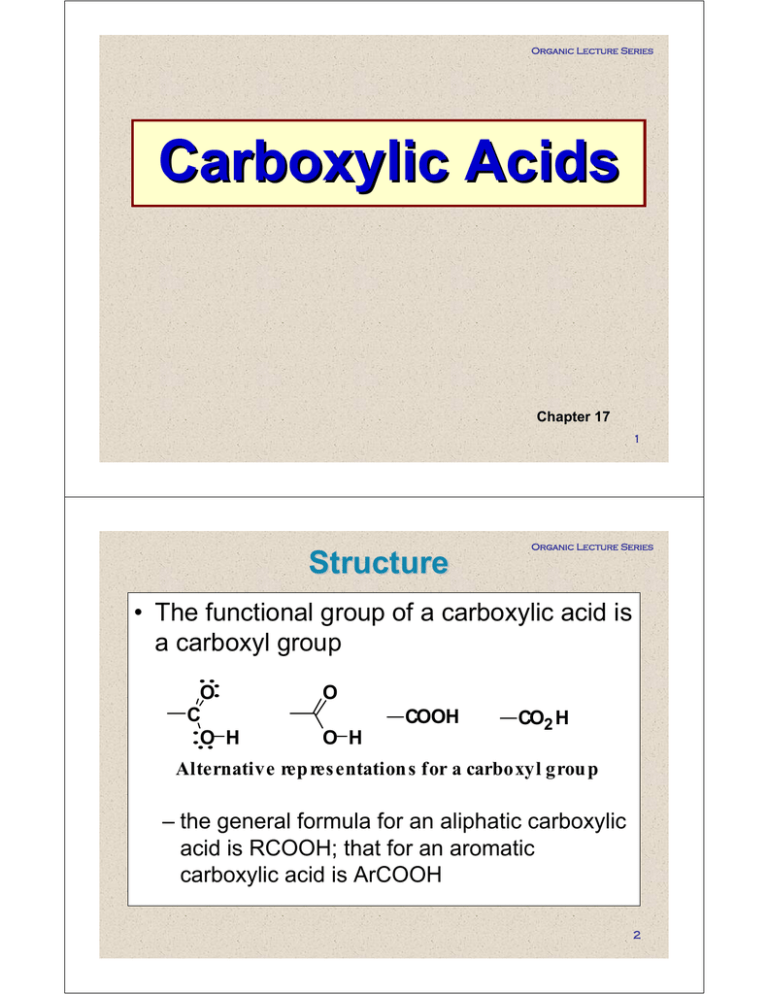
Organic Lecture Series Carboxylic Acids Chapter 17 1 Structure Organic Lecture Series • The functional group of a carboxylic acid is a carboxyl group O C O H O COOH O H CO2 H Alternative rep res entation s for a carboxyl grou p – the general formula for an aliphatic carboxylic acid is RCOOH; that for an aromatic carboxylic acid is ArCOOH 2 Organic Lecture Series Nomenclature • IUPAC names: drop the -e from the parent alkane and add the suffix -oic acid O HCOOH CH3 COOH Methan oic acid (Formic acid ) Eth anoic acid (Acetic acid) OH 3-Methylbutanoic acid (Isovaleric acid ) – if the compound contains a carbon-carbon double bond, change the infix -anan to -enen O O O OH Propen oic acid (A crylic acid) OH OH t rans-2-Butenoic acid (Crotonic acid ) t rans-3-Phen ylp ropen oic acid (Cinnamic acid ) 3 Organic Lecture Series • The carboxyl group takes precedence over most other functional groups OH O O OH (R)-5-Hydroxyhexan oic acid O O OH 5-Oxohexan oic acid H2 N OH 4-Aminobutan oic acid O O δ γ β α 5 1 3 4 2 OH OH OH γ-hydroxybutyric Acid GHB 4 Organic Lecture Series Nomenclature – dicarboxylic acids: add the suffix -dioic acid to the name of the parent alkane containing both carboxyl groups O HO O OH O Ethanedioic acid (Oxalic acid) O HO O OH O Butanedioic acid (Succinic acid) HO O HO OH Propanedioic acid (Malonic acid) O O OH HO OH O Pentanedioic acid (Glutaric acid) Hexanedioic acid (Adipic acid) 5 Organic Lecture Series Nomenclature – if the carboxyl group is bonded to a ring, name the ring compound and add the suffix -carboxylic acid 2 3 1 COOH 2-Cyclohexen ecarboxylic acid HOOC COOH t rans-1,3-Cyclopentan edicarboxylic acid – benzoic acid is the simplest aromatic carboxylic acid – use numbers to show the location of substituents COOH COOH OH COOH COOH COOH COOH Benzoic 2-Hydroxyben zoic 1,2-Benzenedicarb oxylic 1,4-Ben zenedicarboxylic acid acid acid acid (Tereph thalic acid ) (Ph thalic acid ) (Salicylic acid ) 6 Organic Lecture Series Nomenclature – when common names are used, the letters α, β, γ, δ, etc. are often used to locate substituents O δ γ β α 5 1 3 4 2 O O OH H2 N OH 4-A min ob utanoic acid (γ-A min obu tyric acid, GABA) OH NH2 (S)-2-Aminopropanoic acid [(S)-α-Aminop ropionic acid; L-alanine] 7 Organic Lecture Series Physical Properties • In the liquid and solid states, carboxylic acids are associated by hydrogen bonding into dimeric structures 8 Organic Lecture Series Physical Properties • Carboxylic acids have significantly higher boiling points than other types of organic compounds of comparable molecular weight –they are polar compounds and form very strong intermolecular hydrogen bonds 9 Organic Lecture Series Physical Properties • Carboxylic acids are more soluble in water than alcohols, ethers, aldehydes, and ketones of comparable molecular weight –they form hydrogen bonds with water molecules through their C=O and OH groups 10 Organic Lecture Series Physical Properties Molecu lar Boiling Weight Point (°C) (g/mol) Solu bility (g/100 g H 2 O) Structu re Name CH3 COOH Acetic acid 60.1 118 Infinite CH3 CH2 CH2 OH 1-Propan ol 60.1 97 Infinite CH3 CH2 CHO Propan al 58.1 48 16 88.1 88.1 86.1 163 137 103 Infinite 2.3 Slight CH3 ( CH2 ) 4 COOH Hexan oic acid 116.2 CH3 (CH2 ) 5 CH2 OH 1-Hep tanol 116.2 205 1.0 176 CH3 ( CH2 ) 5 CHO 153 0.2 0.1 CH3 ( CH2 ) 2 COOH Butanoic acid CH3 (CH2 ) 3 CH2 OH 1-Pentanol CH3 ( CH2 ) 3 CHO Pentanal Hep tanal 114.1 11 Organic Lecture Series Physical Properties – water solubility decreases as the relative size of the hydrophobic portion of the molecule increases 12 Organic Lecture Series Acidity • Carboxylic acids are weak acids – values of pKa for most aliphatic and aromatic carboxylic acids fall within the range 4 to 5 • The greater acidity of carboxylic acids relative to alcohols (both compounds that contain an OH group) is due to resonance stabilization of the carboxylate anion: O H 3C O O OH H H 3C O H 3C O 13 Acidity Organic Lecture Series – electron-withdrawing substituents near the carboxyl group increase acidity through their inductive effect Formula: CH3 COOH ClCH2 COOH Cl2 CHCOOH Name: Acetic Chloroacetic D ich loroacetic acid acid acid 2.86 pKa : 1.48 4.76 Cl3 CCOOH Trich loroacetic acid 0.70 Increasin g acid strength 14 Organic Lecture Series – the form of a carboxylic acid present in aqueous solution depends on the pH of the solution O O O O OH OH + R-C-O R-C-OH R-C-OH R-C-O + + H H predomin ant present in eq ual p red ominan t species w hen concentrations w hen sp ecies w hen the pH of the the pH of the th e pH of the s olution is solution is solution is eq ual to 2.0 or less 7.0 or greater the pK a of the acid [A-] pH = pKa + log10 [HA] 15 Organic Lecture Series For organic acids in equilibrium: HA + H + A - Henderson-Hasselbalch equation: [A-] pH = pKa + log10 [HA] 16 Reaction with Bases Organic Lecture Series Carboxylic acids, whether soluble or insoluble in water, react with NaOH, KOH, and other strong bases to give watersoluble salts COOH + N aOH - H2 O Benzoic acid (slightly soluble in water) Acid + COO Na + + H2 O Sodium benzoate (60 g/100 mL water) Base + Salt water 17 Reaction with Bases Organic Lecture Series • They also form water-soluble salts with ammonia and amines COOH + NH 3 Benzoic acid (slightly soluble in water) - H2 O COO NH4 + Ammonium benzoate (20 g/100 mL water) 18 Reaction with Bases Organic Lecture Series • Carboxylic acids react with sodium bicarbonate and sodium carbonate to form water-soluble salts and carbonic acid – carbonic acid, in turn, breaks down to carbon dioxide and water CH3 COOH + NaHCO3 H2 CO3 CH3 COOH + NaHCO3 + CH3 COO Na + H2 CO3 CO2 + H2 O - CH3 COO Na + + CO2 + H2 O 19 Organic Lecture Series Preparation • Carbonation of Grignard reagents – treatment of a Grignard reagent with carbon dioxide followed by acidification gives a carboxylic acid O MgBr + C O Carb on dioxid e O C-O - [MgBr] + A magnesium carboxylate HCl H2 O O C-OH + Mg2 + Cyclop entanecarboxylic acid 20 Organic Lecture Series Reduction • The carboxyl group is very resistant to reduction – it is not affected by catalytic hydrogenation under conditions that easily reduce aldehydes and ketones to alcohols, and reduce alkenes and alkynes to alkanes; it is not reduced by NaBH4 • Lithium aluminum hydride reduces a carboxyl group to a 1° alcohol – reduction is carried out in diethyl ether, THF, or other nonreactive, aprotic solvent O COH 1 . LiAlH4 2 . H2 O CH2 OH + LiOH + Al(OH) 3 4-Hydroxymeth ylcyclopenten e 21 Selective Reduction Organic Lecture Series – carboxyl groups are not affected by catalytic reduction under conditions that reduce aldehydes and ketones O OH O OH + H2 5-Oxohexanoic acid Pt 25°C, 2 atm O OH 5-Hydroxyhexan oic acid (racemic) – nor are carboxyl groups reduced by NaBH4 O O OH O 1 . NaBH4 C6 H5 OH 2 . H2 O 5-Oxo-5-ph enylp entanoic acid 5-Hyd roxy-5-phenylpen tanoic acid (racemic) C6 H5 OH 22 Organic Lecture Series Fischer Esterification • Esters can be prepared by treating a carboxylic acid with an alcohol in the presence of an acid catalyst, commonly H2SO4, ArSO3H*, or gaseous HCl O H2 SO 4 OH Ethanoic acid (Acetic acid) + HO Ethanol (Ethyl alcohol) O O Ethyl ethanoate (Ethyl acetate) + H2 O O *H C 3 S OH O T o lu e n e S u lfo n ic A c id TsO H Fischer Esterification 23 Organic Lecture Series Fischer esterification is an equilibrium reaction: by careful control of experimental conditions, it is possible to prepare esters in high yield if the alcohol is inexpensive relative to the carboxylic acid, it can be used in excess to drive the equilibrium to the right alternatively, water can be removed by azeotropic distillation and a Dean-Stark trap 24 Organic Lecture Series Fischer Esterification a key intermediate in Fischer esterification is the tetrahedral carbonyl addition intermediate formed by addition of ROH to the C=O group O TCAI H R C OCH3 O H H+ H+ O R C OH + HOCH3 O R C OCH 3 + HOH Refer to mechanism worksheet 25 Organic Lecture Series Acid Chlorides • The functional group of an acid halide is a carbonyl group bonded to a halogen atom – among the acid halides, acid chlorides are by far the most common and the most widely used O - C- X Functional group of an acid halide O CH 3 CCl A cetyl chloride O C-Cl Benzoyl chloride 26 Organic Lecture Series Acid Chlorides – acid chlorides are most often prepared by treating a carboxylic acid with thionyl chloride O OH Bu tanoic acid O + SOCl2 + SO2 + HCl Cl Th ionyl ch loride Butanoyl chlorid e 27 Organic Lecture Series Acid Chlorides • The mechanism for this reaction is divided into two steps. Step 1: reaction with SOCl2 transforms OH, a poor leaving group, into a chlorosulfite group, a good leaving group O O R-C-O-H + Cl-S-Cl O O R C O S Cl + H-Cl A chloros ulfite grou p 28 Organic Lecture Series Acid Chlorides Step 2: attack of chloride ion gives a tetrahedral carbonyl addition intermediate, which collapses to give the acid chloride O O R C O S Cl + Cl - O O: R C O S Cl O R-C-Cl + SO2 + Cl - Cl A tetrah edral carbonyl ad dition intermed iate Note: the reaction is irreversible 29 Organic Lecture Series Decarboxylation • Decarboxylation: loss of CO2 from a carboxyl group – carboxylic acids, if heated to a very high temperature, undergo thermal decarboxylation O R-C-OH decarb oxylation heat R-H + CO2 – most carboxylic acids, however, are quite resistant to moderate heat and melt or even boil without decarboxylation 30 Organic Lecture Series Decarboxylation The most important exceptions are carboxylic acids that have a carbonyl group beta to the carboxyl group i.e.- β keto-acids –this type of carboxylic acid undergoes decarboxylation on mild heating O O O warm β α OH 3-Oxobu tan oic acid (Acetoacetic acid) + CO2 Acetone 31 Organic Lecture Series Decarboxylation – thermal decarboxylation of a β-ketoacid involves rearrangement of six electrons in a cyclic six-membered transition state O H O (1) O (A cyclic s ix-membered tran sition state) O H O + C O En ol of a ketone (2) O + CO2 A ketone 32 Organic Lecture Series Decarboxylation – decarboxylation occurs if there is any carbonyl group beta to the carboxyl – malonic acid and substituted malonic acids, for example, also undergo thermal decarboxylation O O 140-150°C HOCCH2 COH Prop anedioic acid (Malon ic acid ) O CH3 COH + CO2 33 Organic Lecture Series – thermal decarboxylation of malonic acids also involves rearrangement of six electrons in a cyclic six-membered transition state O HO H O O (1) O HO H O + C O A cyclic six-membered Enol of a transition s tate carb oxylic acid (2) O HO A carb oxylic acid + CO2 34