Hybridization in Chemistry: sp, sp2, sp3 Orbitals Explained
advertisement
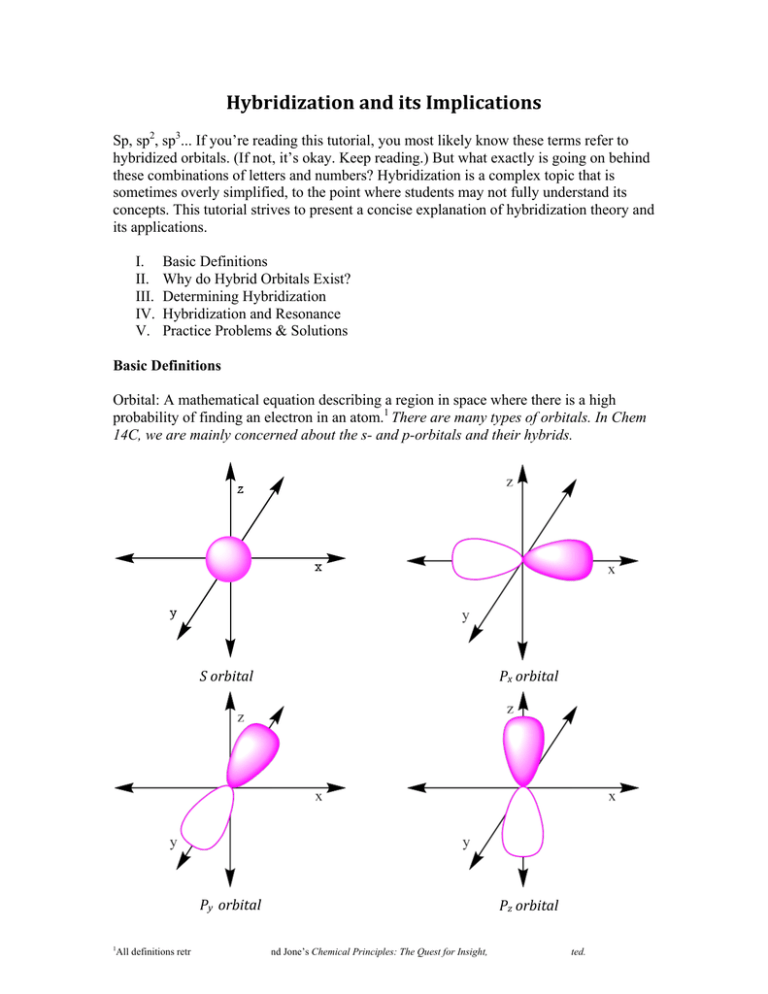
Hybridization and its Implications Sp, sp2, sp3... If you’re reading this tutorial, you most likely know these terms refer to hybridized orbitals. (If not, it’s okay. Keep reading.) But what exactly is going on behind these combinations of letters and numbers? Hybridization is a complex topic that is sometimes overly simplified, to the point where students may not fully understand its concepts. This tutorial strives to present a concise explanation of hybridization theory and its applications. I. II. III. IV. V. Basic Definitions Why do Hybrid Orbitals Exist? Determining Hybridization Hybridization and Resonance Practice Problems & Solutions Basic Definitions Orbital: A mathematical equation describing a region in space where there is a high probability of finding an electron in an atom.1 There are many types of orbitals. In Chem 14C, we are mainly concerned about the s- and p-orbitals and their hybrids. 1 S orbital Px orbital Py orbital Pz orbital All definitions retrieved from Atkins and Jone’s Chemical Principles: The Quest for Insight, unless otherwise stated. As shown above, the s-orbital is sphere-shaped, while the three p-orbitals each consist of two lobes and can be shown as being aligned perpendicularly to each other as if on the x, y, and z axes of a graph. Bonds are formed when the orbitals of multiple atoms overlap. So what’s all the fuss over hybridized orbitals? Why aren’t the normal s- and p-orbitals sufficient? As we are about to see, without hybridized orbitals, many of the atomic bonds we chemistry students take for granted would not exist. Why do hybrid orbitals exist? Atomic orbital: A mixed orbital formed by blending together atomic orbitals on the same atom. Chemist Linus Pauling developed hybridized orbital theory by studying the structure of molecules such as methane (CH4). To answer this question, we will follow in his footsteps. Molecular orbital theory tells us that the C-H bonds in methane are formed by the overlap of each hydrogen s-orbital with carbon’s 2s-orbital and px, py, and pz orbitals. But there’s a problem with this assumption. We know (as Pauling did) that the structure of CH4 is tetrahedral, and that its true bond angles are 109.5°. Carbon’s p-orbitals are lined up so that they are perpendicular, at 90° angles from each other. So how are these tetrahedral s/p-orbital overlaps possible? Bonding with normal orbitals would result in unstable 90° angles between the C-H bonds. Bonding with hybridized orbitals results in the correct 109.5° tetrahedral bond angles. They aren’t. Atomic p-orbitals are fixed in their perpendicular position. Pauling concluded that the only way for methane’s C-H bonds to have 109.5° an angle is if the bonds were hybridized. By blending together its s- and p- orbitals, carbon can break the restrictions of its perpendicular p-orbitals and expand the angle of its bonds to 109.5°. At this angle, the atoms and bonds of methane are farthest apart, meaning that it is in the lowest energy state possible. As with countless concepts in organic chemistry, the driving force behind hybridization is stability (i.e. minimization of energy). Geometry is a key factor in molecular stability, so it is important to remember that hybridization is influenced by geometry, and not the other way around. Without hybridization, the molecules that are the fundamental building blocks of our world would be much more unstable. The number of bonds an atom makes in a molecule determines the amount of hybridized orbitals it needs. In the case of methane, carbon combines its one s-orbital and its three porbitals into four sp3 orbitals in order to bond to the four hydrogen atoms. The hybridized orbitals encountered in Chem 14C also include sp and sp2. Their compositions are shown in the table below. Table I: Hybrid Orbital Composition Hybrid Orbital Orbitals Combined Resulting Orbitals sp s-orbital + 1 p-orbital 2 sp orbitals + 2 p-orbitals sp2 s-orbital + 2 p-orbitals 3 sp2 orbitals + 1 p-orbital sp3 s-orbital + 3 p-orbitals 4 sp3 orbitals (no p-orbitals) It is important to note that the sp3 hybridization leaves no p-orbitals left. This lack of free p-orbitals has large implications, which will be discussed later. Now that we have an understanding of how hybridized orbitals function and why they are necessary, let’s proceed to their application. Determining Hybridization How can we tell what hybrid orbitals an atom needs to form the most stable bonds? Generally, it is as simple as counting the regions of electron density around each atom in order to determine how many orbitals we need in order to form the appropriate number of bonds. In the case of methane, we knew that carbon needed four hybridized orbitals in order to bond to accommodate all four hydrogen atoms; looking at the table above, we see that only sp3 hybridization gives us the necessary amount of hybrid orbitals to form four bonds. A second table condensing this information to a glance has been compiled below. Table II: General Hybridization Scheme Number of Regions of Electron Density Hybridization Shape 2 sp Linear 3 sp2 Trigonal planar 4 sp3 Tetrahedral 5 dsp3 * Trigonal pyramidal 6 d2sp3 * Octahedral *Atoms that are able to accommodate an expanded octet, such as silicon, phosphorus, and sulfur, hybridize their d-orbitals along with their s- and - orbitals. You probably will not work with these hybridizations in 14C, but it is worth knowing. Procedure for determining hybridization of an atom: Step 1: Determine the number of regions of electron density around the atom. Regions of electron density are either bonds or lone pairs. Double and triple bonds each count as just one region. Step 2: Match the number of regions with the corresponding hybridization. Step 3: If hybridization is determined to be sp3, look for any possible resonance that signifies that the atom is in fact sp2-hybridized (this step will be covered in depth in the next section.) Example #1: Determine the hybridization of the indicated atoms. This tutorial assumes that you are familiar with the use and interpretation of line structures. Solution: Looking at the oxygen atom, we see that is has a double bond and two lone pairs. Therefore we count three regions of electron density around the oxygen atom (remember, a double bond counts as just one region), and, using the table, conclude that the oxygen atom is sp2 hybridized. Looking at carbon now, there seem to be just two attachments, but we should recognize that this is a line structure and there are two hydrogen bonds not shown. Taking this into account, we count 4 regions of electron density and conclude carbon is sp3 hybridized. The chlorine has 3 lone pairs and one bond. 3 + 1 = 4, and we conclude that chlorine is also sp3 hybridized. The next step would be to check for resonance, but we will discuss this in detail next. This molecule, for the record, does not have resonance, so these hybridizations are correct. A note on good practice: If you are given a question on the hybridization of an atom but you are not given a line structure for it, draw a Lewis structure. You may be able to contemplate the hybridization mentally, but if there are other things at work such as resonance, your surest bet is to draw the structure. We have the general method down now, but you should keep reading. In order to master hybridization, we must know how to recognize when an “sp3” hybridized atom participates in resonance. Hybridization and Resonance Resonance: a condition in which one Lewis structure is not sufficient to represent a molecule; multiple Lewis structures are needed.2 Hybridization’s crucial exception is this: when an atom that appears to be sp3 hybridized can participate in resonance, that atom is in fact sp2 hybridized. This is because resonance requires an open p-orbital to occur. Sp3 atoms have 4 sp3-hybridized orbitals but no open p-orbitals, and therefore cannot participate in resonance. Sp2-hybridized atoms can. Why is it important for resonance to occur? Again, the answer lies in stability. Structures with resonance are more stable than similar structures without resonance, so whenever an atom has an opportunity to free up a p-orbital to accommodate resonance, it will do so. How often will this exception come up? Pretty often. In fact, almost continuously. Mastery of important Chem 14C topics such as conjugation and aromaticity depend on your ability to discern an sp2 atom from an sp3 one. Let us work through an example. 2 This definition is derived from Dr. H’s Illustrated Glossary of Organic Chemistry. Example 2: Determine the hybridization of the indicated atoms. Solution: The upper oxygen atom has three attached regions of electron density, and we conclude that it is sp2 hybridized. Turning to the lower oxygen, we count four regions of electron density, and conclude that it is sp3 hybridized. But before we leave this as our final answer, we need to investigate the possibility that this oxygen can participate in resonance. A note on good practice: Drawing curve arrows is very helpful when drawing resonance contributors. Drawing this molecule’s only other significant resonance contributor shows that this oxygen definitely participates in resonance. In order for this resonance to occur, this oxygen atom must have an open p-orbital, and we therefore conclude that it is sp2 hybridized. A note on good practice: When looking for other resonance structures, keep in mind that definite sp3 orbitals (such as a carbon with four bonds) prevent resonance from occurring through them due to their lack of a p-orbital. P-orbitals are like bridges that electrons use as a path for resonance (delocalization). If there is no bridge, the path cannot be traveled. Practice Problems Determine the hybridization of the indicated atoms. To be consistent with the problems encountered in 14C, lone pairs and most hydrogen atoms are not shown. 1. 2. 3. 5. 7. 4. 6. 8. 9. 10. 11. 12. 13. 14. Practice Problem Solutions Circled in red are certain sp3 atoms that prevent resonance from occurring. Many resonance contributors involve a negative formal charge on carbon. 1. sp3 2. sp2 – resonanceabled sp2 sp3 3. 4. sp3 sp2 – resonance -abled sp2 5. 6. sp3 sp 7. sp2 – resonance-abled 9. sp2 – resonance-abled 8. sp2 – resonance-abled sp3 10. sp3 sp3 2 sp – resonance-abled Lone pairs are surrounded by sp3 atoms, no available porbitals for resonance. 11. sp3 sp2 – resonanceabled sp3 13. 12. sp3 sp All these atoms have lone pairs, but nowhere to move them. Verify this by trying to use curved arrows to make something happen, without breaking the octet rule. Highly electronegative atoms such as chlorine are still resonance participators. sp2 – resonanceabled 3 14. sp3 sp2 – resonanceabled The carbon in red is still The carbon with the + sign sp3. has three attachments, only and an open p-orbital.







