Balancing Equations
advertisement

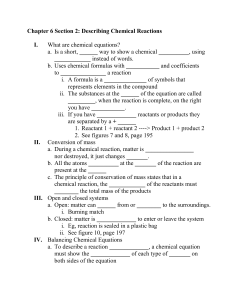
BALANCING EQUATIONS AND TYPES OF CHEMICAL REACTIONS What is a Chemical Equation? An equation is a short-hand way of writing a chemical reaction. Reactants are on the left, products are on the right. Mg + 2HCl MgCl2 + H2 reactants products How is this Accomplished? Chemical equations are balanced by adding coefficients in front of the substances in the equation. 1 MgF2 + 1 Li2CO3 1 MgCO3 + 2 LiF Never change the subscript to balance equations Tips for Balancing Equations 1. Balance metals first. 2. Balance nonmetals except H and O. 3. If polyatomic ions are conserved in the reaction, then try balancing them as a unit. 4. Balance any remaining H’s and O’s. Some practice problems: Here are some practice problems. 1. __NaCl + __BeF2 --> __NaF + __BeCl2 2NaCl + BeF2 2NaF + BeCl2 2. __FeCl3 + __Be3(PO4)2 --> __BeCl2 + __FePO4 2FeCl3 + Be3(PO4)2 --> 3BeCl2 + 2FePO4 3. __AgNO3 + __LiOH --> __AgOH + __LiNO3 AgNO3 + LiOH --> AgOH + LiNO3 4. __CH4 + __O2 --> __CO2 + __H2O CH4 + 2O2 --> CO2 + 2H2O 5. __Mg + __Mn2O3 --> __MgO + __Mn 3Mg + Mn2O3 --> 3MgO + 2Mn 7. Na + H2O NaOH + H2 2Na + 2H2O 2NaOH + H2 8. H2SO4 + Ca(OH)2 CaSO4 + H2O H2SO4 + Ca(OH)2 CaSO4 +2 H2O Types of Chemical Reactions There are 5 overall types of chemical reactions: 1. Synthesis or Combination 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion Synthesis: A + B AB Synthesis – “putting together” H2(g) + Cl2(g) ----> 2HCl(g) C(s) + O2(g) ----> CO2(g) CaO(s) + H2O(l) ----> Ca(OH)2(s) Decomposition: AB A + B Decomposition – “breaking apart” C12H22O11(s) ---->12C(s) + 11H2O(g) Pb(OH)2(s) ----> PbO(s) + H2O(g) 2Ag2O(s) ----> 4Ag(s) + O2(g) Single Replacement: A+ BC B + AC Single replacement reactions - When one element displaces another element in a compound Zn(s) + H2SO4(aq) ----> ZnSO4(aq) + H2(g) 2Al(s) + 3CuCl2(aq) ---> 2AlCl3(aq) + 3Cu(s) Cl2(g) + KBr(aq) ----> KCl(aq) + Br2(l) Double Replacement: AB + CD AD + CB Double Replacement reaction – chemical reaction where two reactant ionic compounds exchange ions to form two new product compounds with the same ions. AgNO3(aq) + NaCl(aq) ----> AgCl(s) + NaNO3(aq) ZnBr2(aq) + 2AgNO3(aq) ----> Zn(NO3)2(aq) + 2AgBr(s) H2SO4(aq) + 2NaOH(aq) ----> Na2SO4(aq) + 2H2O(l) Combustion: Burning (add O2) CH4(g) + 2O2(g) ----> 2H2O(g) + CO2(g) C2H6(g) + O2(g) ----> H20(g) + CO2(g) C3H8(g) + O2(g) ----> H2O(g) + CO2(g) H2(g) + O2(g) ----> H2O(g) Mg(g) + O2(g) ----> MgO(s)