Chapter 6 and 19 AP Review
advertisement

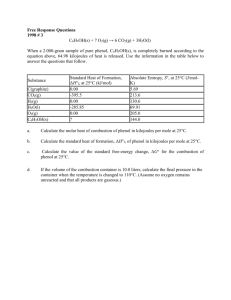
1) Which of the following is probably true for a solid solute with a highly endothermic heat of solution when dissolved in water? a.The solid has a low lattice energy. b.As the solute dissolves, the temperature of the solution increases. c.The resulting solution is ideal. d.The solid is more soluble at higher temperatures. e.The solid has a high energy of hydration. D 2). Which of the following reactions has the largest positive value of ΔS per mole of Cl2. a. H2(g) + Cl2(g) --> 2 HCl(g) b. C12(g) + 2 O2(g) --> Cl2O(g) c. Mg(s) + Cl2(g) --> MgCl2(s) d. 2 NH4Cl(s) --> 4 H2(g) + Cl2(g) e. Cl2(g) --> 2 Cl(g) D 3). Which of the following must be true for a reaction that proceeds spontaneously from initial standard state conditions? a. ΔG° > 0 and Keq > 1 b. ΔG° > 0 and Keq < 1 c. ΔG° < 0 and Keq > 1 d. ΔG° < 0 and Keq < 1 e. ΔG° = 0 and Keq = 1 • C 4) H2O(s) --> H2O(l) When ice melts at its normal melting point, 273.16 K and 1 atmosphere, which of the following is true for the process shown above? a. DH < 0, DS > 0, DV > 0 b. DH < 0, DS < 0, DV > 0 c. DH > 0, DS < 0, DV < 0 d. DH > 0, DS > 0, DV >0 e. DH > 0, DS > 0, DV < 0 E 5) CH4(g) + 2 O2(g) --> CO2(g) + 2 H2O(l) ΔHf°CH4 = –889.1 kJ ΔHf°H2O(l) = –285.8 kJ/mol ΔHf°CO2(g) = –393.3 kJ/mol What is the standard heat of formation of methane, ΔHf°CH4(g), as calculated from the data above? (A) –210.0 kJ/mole (D) 75.8 kJ/mole (B) –107.5 kJ/mole (E) 210.0 kJ/mole (C) –75.8 kJ/mole c ΔH° = –286 kJ 2 Na(s) + 1/2 O2(g) --> Na2O(s) ΔH°= –414 kJ Na(s) + 1/2 O2(g) + 1/2 H2(g) --> NaOH(s) ΔH°= – 425 kJ 6) H2(g) + 1/2 O2(g) --> H2O(l) Based on the information above, what is the standard enthalpy change for the following reaction? Na2O(s) + H2O(l) --> 2 NaOH(s) A) –1,125 kJ (B) –978 kJ (C) –722 kJ (D) –150 kJ (E) +275 kJ • D 7) For which of the following processes would ΔS have a negative value? I. 2 Fe2O3(s) --> 4 Fe(s) + 3 O2(g) II.Mg2+ + 2 OH– --> Mg(OH)2(s) III. H2(g) + C2H4(g) --> C2H6(g) (A) I only (B) I and II only (C) I and III only (D) II and III only (E) I, II, and III • D 8) N2(g) + 3 H2(g) --> 2 NH3(g) The reaction indicated above is thermodynamically spontaneous at 298 K, but becomes nonspontaneous at higher temperatures. Which of the following is true at 298 K? (A) ΔG, ΔH, and ΔS are all positive. (B) ΔG, ΔH. and ΔS are all negative. (C) ΔG and ΔH are negative, but ΔS is positive. (D) ΔG and ΔS are negative, but ΔH is positive. (E) ΔG and ΔH are positive, but ΔS is negative. • B 1997 D For the gaseous equilibrium represented below, it is observed that greater amounts of PCl3 and Cl2 are produced as the temperature is increased. PCl5(g) ® PCl3(g) + Cl2(g) (a) What is the sign of DS° for the reaction? Explain. (b) What change, if any, will occur in DG° for the reaction as the temperature is increased? Explain your reasoning in terms of thermodynamic principles. (c) If He gas is added to the original reaction mixture at constant volume and temperature, what will happen to the partial pressure of Cl2? Explain. (d) If the volume of the reaction mixture is decreased at constant temperature to half the original volume, what will happen to the number of moles of Cl2 in the reaction vessel? Explain. 1996 B C2H2(g) + 2 H2(g) C2H6(g) Information about the substances involved in the reaction represented above is summarized in the following tables. Substance C2H2(g) H2(g) C2H6(g) S° (J/molK) DH°f (kJ/mol) 200.9 226.7 130.7 0 xx -84.7 • (a) If the value of the standard entropy change, DS°, for the reaction is -232.7 joules per moleKelvin, calculate the standard molar entropy, S°, of C2H6 gas. • (b) Calculate the value of the standard free-energy change, DG°, for the reaction. What does the sign of DG° indicate about the reaction above? • (c) Calculate the value of the equilibrium constant, K, for the reaction at 298 K. ( 1998 B C6H5OH(s) + 7 O2(g) 6 CO2(g) + 3 H2O(l) When a 2.000-gram sample of pure phenol, C6H5OH(s), is completely burned according to the equation above, 64.98 kilojoules of heat is released. Use the information in the table below to answer the questions that follow. Substance Standard Heat of Formation Absolute Entropy DH°C at 25°C (kJ/mol) , S°, at 25°C(J/mol K) C(graphite) 0.00 5.69 CO2(g) -393.5 213.6 H2(g) 0.00 130.6 H2O(l) -285.85 69.91 O2(g) 0.00 205.0 C6H5OH(s) ? 144.0 • (a) Calculate the molar heat of combustion of phenol in kilojoules per mole at 25°C. • (b) Calculate the standard heat of formation, DH°, of phenol in kilojoules per mole at 25°C. • (c) Calculate the value of the standard free-energy change, DG°, for the combustion of phenol at 25°C. • (d) If the volume of the combustion container is 10.0 liters, calculate the final pressure in the container when the temperature is changed to 110.°C. (Assume no oxygen remains unreacted and that all products are gaseous.)