AP Chemistry Exam Questions: Thermochemistry & Equilibrium
advertisement
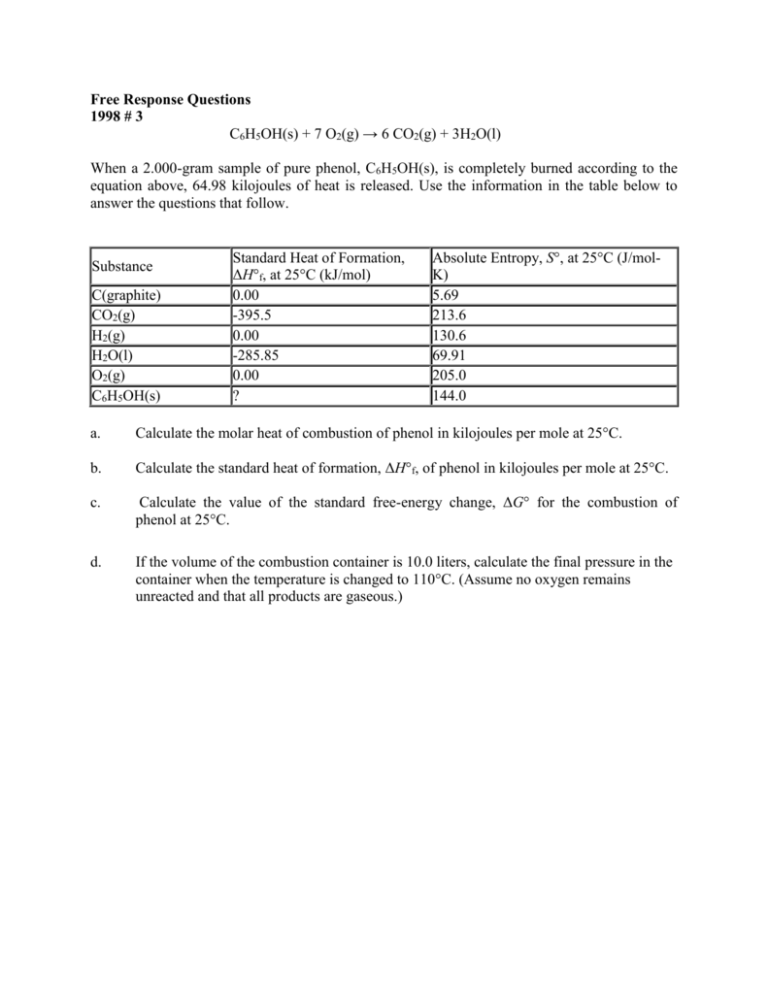
Free Response Questions 1998 # 3 C6H5OH(s) + 7 O2(g) → 6 CO2(g) + 3H2O(l) When a 2.000-gram sample of pure phenol, C6H5OH(s), is completely burned according to the equation above, 64.98 kilojoules of heat is released. Use the information in the table below to answer the questions that follow. Substance C(graphite) CO2(g) H2(g) H2O(l) O2(g) C6H5OH(s) Standard Heat of Formation, ΔH°f, at 25°C (kJ/mol) 0.00 -395.5 0.00 -285.85 0.00 ? Absolute Entropy, S°, at 25°C (J/molK) 5.69 213.6 130.6 69.91 205.0 144.0 a. Calculate the molar heat of combustion of phenol in kilojoules per mole at 25°C. b. Calculate the standard heat of formation, ΔH°f, of phenol in kilojoules per mole at 25°C. c. Calculate the value of the standard free-energy change, ΔG° for the combustion of phenol at 25°C. d. If the volume of the combustion container is 10.0 liters, calculate the final pressure in the container when the temperature is changed to 110°C. (Assume no oxygen remains unreacted and that all products are gaseous.) 1996 #3 C2H2(g) + 2 H2(g) → C2H6(g) Information about the substances Substance S° (J/mol K) ΔH°f (kJ/mol) Bond Bond Energy (kJ/mol) C2H2(g) H2(g) C2H6(g) 226.7 0 -84.7 C-C C=C C-H H-H 347 611 414 436 200.9 130.7 -------- a. If the value of the standard entropy change, ΔS°, for the reaction is -232.7 joules per mole Kelvin, calculate the standard molar entropy, S°, of C2H6 gas. b. Calculate the value of the standard free-energy change, ΔG°, for the reaction. What does the sign of ΔG° indicate about the reaction above? c. Calculate the value of the equilibrium constant, K, for the reaction at 298 K. (Can’t do this part yet) d. Calculate the value of the C ≡C bond energy in C2H2 in kilojoules per mole. 1997 #7 For the gaseous equilibrium represented below, it is observed that greater amounts of PCl3 and Cl2 are produced as the temperature is increased. PCl5(g) PCl3(g) + Cl2(g) a. What is the sign of ΔS° for the reaction? Explain. b. What change, if any, will occur in ΔG° for the reaction as the temperature is increased. Explain your reasoning in terms of thermodynamic principles. c. If He gas is added to the original reaction mixture at constant volume and temperature, what will happen to the partial pressure of Cl2? Explain. d. If the volume of the original reaction is decreased at constant temperature to half the original volume, what will happen to the number of moles of Cl2 in the reaction vessel? Explain. Key 1) 9 points 1 mol 0.02125 mol phenol 1 point 94.113 g 64.98 kJ 3,058 kJ mol 1 Heat released per mole 0.02125 mol Or, ΔHcomb = -3058 kJ mol-1 1 point Units not necessary b) ΔHcomb = -3058 kJ mol-1 1 point -3058 kJ = [6(-395.5) + 3(-285.85)] – [ΔHfo (phenol)] 1 point o ΔHf (phenol) = -161 kJ 1 point One point earned for correct sign of heat of combustion, one point for correct use of moles / coefficients, and one point for correct substitution c) ΔSo = [3(69.91) + 6(213.6)] [7(205.0) + 144.0] = -87.67 J/K 1 point o o o -1 ΔG = ΔH - TΔS = 3058 kJ – (298 K)(-0.08767 kJ K ) = -3032 kJ 1 point Units not necessary; no penalty if correct except for wrong ΔH comb for part a d) moles gas = 9 × [moles from part a] = 9 (0.02125 mol) = 0.1913 moles gas nRT (0.1913 mol)(0.0821 L atm mol 1 K 1 )(383 K ) P 0.601 atm 1 point V 10.0 L Units necessary; no penalty for using Celcius temperature if also lost point in part c for same error a) 2.000 g 2) 9 points (a) two points; one for line of answer - 232.7 J/K = S° (C2H6) - (261.4 + 200.9) J./K S° (C2H6) = 229.6 J/K units ignored; 1 point earned for 98.9 J/K; 1 point lost if stoichiometry is not implied in process (b) three points total; one point each portion; any value for T (e.g., 273 K or 298 K) is allowable: ΔH° = (- 84.7 kJ) - (226.7 kJ) = -311.4kJ ΔG°= - 311.4kJ - (298 K) (- 0.2327kJ/K) ΔG°= - 311.4 kJ + 69.3 kJ ΔG°= - 242.1 kJ Negative ΔG° therefore reaction is spontaneous, or Keq > 1 therefore reaction is spontaneous, or products are favored at equilibrium. (c) two points ln K = 242.1 ÷ [(8.31 × 10-3) (298)] = 97.7 K = 3 × 1042 (1,2,or 3 significant figures acceptable) (d) two points; first point earned for correct substitution and correct number of bonds, second point earned for setting equal to ΔHrxn and correct calculation of answer; no points earned for "extrapolation" techniques to find carbon-carbon triple bond energy; E* is the energy of the carbon-carbon triple bond. - 311.4 kJ = [(2) (436) + E* + (2) (414)] - [(347) + (6) (414)] E* = 820 kJ 5 points 3) (a) S° is positive (or "+", or ">0") 1 point Moles products > moles reactants 1 point Note; all species are gaseous, so (g) need not be indicated. To earn credit, number of particles (moles) must be discussed. No explanation point earned for just noting that disorder increases, or that PCl5 is decomposing or dissociating. (b) G° will decrease (or become more negative, or become smaller). 1 point G° = H° - TS° and since S° is positive, TS° is positive ( > 0). Thus increasing T will result in a larger term being subtracted from H°, or, G° = -RT ln K and K is going up in value since T is increasing.) Note: full credit earned for part (b) if: S° < 0 in part (a) which leads to G° is increasing because TS° is added to H°, or, S° = 0 in part (a) which leads to no change in G° (c) no change (one point) PHe is not part of the a) reaction (He is not involved) or, b) law of mass action or, c) reaction quotient or, d) equilibrium constant expression; one point hence altering PHe has no effect on the position at equilibrium (d) moles of Cl2 will decrease (one point) The decrease in volume leads to an increase in pressure (concentration), therefore the reaction shifts to the left because: (one point for any of the following) Q > Ksp (Q > Kc, or, the rate of the reverse reaction increase more than the rate of the forward reaction, or, the reaction shifts toward the lesser moles of gas. Note: "LeChatelier's principle" alone is not sufficient to earn the explanation point. If response suggests that the number of moles of Cl2 is halved because the system is "cut" in half, only one point is earned.











