Periodic Trends
advertisement

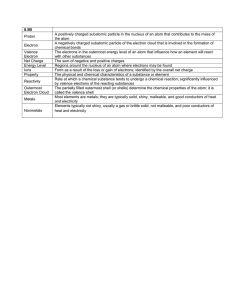
HONORS CHEMISTRY September 18-19, 2013 How do we know what the filling order is? What chemistry tool might we rely on? (Stop) Electron Configurations and the Periodic Table Valence electron configurations repeat down a group Ground state electron configurations Example: Li atomic number = 3 nucleus has 3 protons neutral atom has 3 electrons 2 electrons in 1s orbital, 1 electron in 2s orbital 2s 1s Different ways to show electron configuration Energy level diagram Box notation 1s 2s 2s 1s Spectroscopic notation Li 1s2 2s1 Read this “one s two” not “one s squared” Write the superscript 1. Don’t leave it blank Using the Periodic Table The last subshell in the electron configuration is one of these (row #) s (row # – 1) d (row #) p (row # – 2) f The f-block is inserted into to the dblock Electron configuration of O Atomic number of O = 8 so neutral atom has 8 e– Electron configuration of Co Atomic number of Co = 27 so neutral atom has 27 e– Simplifying electron configurations Shorthand Noble Gas Configuration Build on the atom’s noble gas core He 1s2 O 1s22s22p4 O [He]2s22p4 Ar 1s22s22p63s23p6 Co 1s22s22p63s23p64s23d7 Co [Ar]4s23d7 1s 2s 2p 1s 2s 2p 3s 3p 4s 3d Noble Gases Far right of the periodic table These elements are extremely unreactive or inert They rarely form compounds with other elements Noble Gas electron configurations What is the electron configurations for Neon Abbreviated way to write configurations Start Br Ba with full outer shell then add on Noble Gases Neon- emits brilliant light when stimulated by electricity – neon signs- 4th most abundant element in the universe. Helium- light non reactive gas- used balloonsinexpensive, plentiful and harmless Radon- radioactive gas- can cause cancercolorless, odorless emitted from for certain rocks underground Why are we doing all of this? Properties of atoms correlate with the number and energy of electrons Electron configurations are used to summarize the distribution of electrons among the various orbitals Electron configuration of ions What is an ion? How many electrons does Cl1- have? What is the electron configuration for the chloride ion? How many electrons does Ca2+ have? What is the electron configuration for the calcium ion? What do you notice? Why is this important Valence electrons Electrons in the outermost energy level Where all the action occurs The f-block is inserted into to the dblock Find the electron configuration of Au Locate Au on the periodic table Find the electron configuration of Au Au [Xe] The noble gas core is Xe Find the electron configuration of Au Au [Xe]6s2 The noble gas core is Xe From Xe, go 2 spaces across the s-block in the 6th row 6s2 Find the electron configuration of Au Au [Xe]6s24f14 The noble gas core is Xe From Xe, go 2 spaces across the s-block in the 6th row 6s2 Then detour to go 14 spaces across the f-block 4f14 note: for the f-block, n = row – 2 = 6 – 2 = 4 Find the electron configuration of Au Au [Xe]6s24f145d9 The noble gas core is Xe From Xe, go 2 spaces across the s-block in the 6th row 6s2 Then detour to go 14 spaces across the f-block 4f14 note: for the f-block, n = row – 2 = 6 – 2 = 4 Finally go 9 spaces into the d-block on the 6th row 5d9 note: for the d-block, n = row – 1 = 6 – 1 = 5 Practice Draw the orbital diagram for sulfur. What ion does sulfur want to form and why? Draw the orbital diagram for Potassium. What ion does sulfur want to form and why? What does this mean Properties of atoms correlate with the number and energy of electrons Atoms like to have full outer shells. Refer to Atomic Structure Worksheet Periodic Trends Preview 4 Periodic Trends Atomic Size/Radius Ionic Size (**) Ionization Energy Electronegativity 2 main factors affect periodic trends Number of electron shells (group) Effective Core Charge (ECC) (period) Term (Refer to Definition Sheet) Effective Core Charge (ECC) 1) The net charge that pulls on the valence electrons in an atom. The greater the effective core charge, the greater the pull. It is determined by subtracting the number of core electrons from the number of protons in the nucleus For example: Magnesium (label ECC on P.T.) Term Electron Shell Pattern across the period? Pattern down the group? Periodic Trends Atomic radius The distance from the center of an atoms nucleus to it’s outermost electron Measure of atomic size Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? Atomic radius Periodic Trends Atomic Radius Group Trend Increases from top to bottom More energy levels or quantum levels (or “shell”) as you go down a group – atomic radius increases Period Trend Increases from right to left All electrons in the same energy level. Increased # of protons holds them closer to nucleus. Decrease in Effective Core (Nuclear) Charge (ECC) Calculate ECC for elements in period 2 Table of Atomic Radii Period Trend: Atomic Radius Periodic Trends Ionic Size Size of an atom when electrons are added or removed. Electrons removed atom becomes smaller. Electrons added atoms become larger Why? Electron-Electron Repulsion Ionic Size Cations Positively charged ions formed when an atom of a metal loses one or more electrons Smaller than the corresponding atom Negatively charged ions formed when nonmetallic atoms gain one Anions or more electrons Larger than the corresponding atom Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? Ionic Size (label P.T.) Table of Ion Sizes Ionic Size Group Trend Increases from top to bottom More energy levels as you go down a group – ionic size increases Period Trend Decreases as atoms lose more electrons Increases dramatically as atoms start gaining electrons, decreases as atoms gain fewer electrons. Periodic Trends Ionization Energy Energy needed to remove one of the electrons on an atom’s outer shell. How strongly does an atom hold it’s outermost electron. Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? Ionization Energy Ionization Energy Group Trends Increases from bottom to top. The closer outer shell electrons are to the nucleus the harder they are to remove. Period Trend Increases from left to right. The more electrons in the outer shell the harder it is to remove one. Increase in Effective Core Charge (ECC) Periodic Trend: Ionization Energy Periodic Trends Electronegativity Is a measure of the level of attraction (pull) an atom exerts on the electrons of another atom. Ability of an atom to attract electrons Which elements want to gain electrons the most? Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? Electronegativity Periodic Table of Electronegativities Electronegativity Group Trend Increases from bottom to top As radius decreases, electrons are closer to the nucleus (decrease in number of electron shells) Period Trend Increases from left to right The more electrons in the outer shell (up to 7) the more the atom wants to attract electrons Exception: Trend does not apply to Noble Gases Increase in Effective Core Charge (ECC) Periodic Trend: Electronegativity Summarize the Trends Questions??? Summary of Periodic Trends Practice 1. Se and Br 1. 2. 2. P, S, Se 1. 2. 3. Largest atom Highest Ionization Energy Cl, Cl1-, Br, Br11. 4. Smallest atom Lowest Ionization Energy Largest ionic size Mg, Mg2+, Na, Na1+ 1. Smallest ionic size Atomic Properties Definitions For Quiz – Monday Effective Core Charge: It is the net charge that pulls on the valence electrons in an atom. The greater the effective core charge, the greater the pull. It is determined by subtracting the number of core electrons from the number of protons in the nucleus Valence Electrons Are found in the outermost, valence, electron shell (Bohr model) of the atom Core electrons occupy all of the inner electron, core, shells Atomic Properties Definitions Ionization Energy: Atomic size Energy needed to remove an electron from an atom or molecule. The higher the effective core charge and lower the number of electrons shells, the greater the ionization energy How big (e.g., radius) an atom is Atomic radius is measured from the center of the nucleus to the valence electron shell. The higher the effective core charge and lower the number of electron shells, the smaller the atom. Electronegativity Measure of the level of attraction (pull) an atom exerts on the electrons of another atom. The higher the effective core charge and lower the number of electron shells, the greater the electronegativity Homework Atomic Structure Worksheet 5-3 Worksheet Study for Definition Quiz on Monday Periodic Table Objective: Students know how to use the periodic table to identify alkali metals, alkaline earth metals, transition metals, metals, semimetals (metalloids), nonmetals, halogens and noble gases. The Periodic Table Dmitri Mendeleev – credited for the first periodic table in 1869. He had put element names and a few of their properties on cards and then arranged them in various ways to help his students learn them more easily. Arranged them so elements in the same column have similar properties. Reactivity: Metal/NonMetal Trends ELEMENT CLASSES Periodic Song: http://www.privatehand.com/flash/elements.html Reading the periodic table Groups or families – vertical columns Periods – horizontal rows Alkali Metals All alkali metals have 1 valence electron They are very reactive Reactivity of these elements increases down the group Alkali metals: Potassium, K reacts with water and http://video.google.com/videopl must be stored in ay?docid=kerosene 2134266654801392897# Alkaline Earth Metals All alkaline earth metals have 2 valence electrons Alkaline earth metals are less reactive than alkali metals The word “alkaline” means “basic” common bases include salts of the metals Ca(OH)2 Mg(OH)2 Properties of Metals Metals are good conductors of heat and electricity Metals are malleable Metals are ductile Metals have high tensile strength Metals have luster Transition Metals Copper, Cu, is a relatively soft metal, and a very good electrical conductor. Mercury, Hg, is the only metal that exists as a liquid at room temperature Properties of Metalloids They have properties of both metals and nonmetals. Metalloids are more brittle than metals, less brittle than most nonmetallic solids Metalloids are semiconductors of electricity Some metalloids possess metallic luster Silicon, Si – A Metalloid Silicon has metallic luster Silicon is brittle like a nonmetal Silicon is a semiconductor of electricity Other metalloids include: Boron, B Germanium, Ge Arsenic, As Antimony, Sb Tellurium, Te Nonmetals Nonmetals are poor conductors of heat and electricity Nonmetals tend to be brittle Many nonmetals are gases at room temperature Carbon, the graphite in “pencil lead” is a great example of a nonmetallic element. Examples of Nonmetals Sulfur, S, was once known as “brimstone” Graphite is not the only pure form of carbon, C. Diamond is also carbon; the color comes from impurities caught within the crystal structure Microspheres of phosphorus, P, a reactive nonmetal Halogens Halogens all have 7 valence electrons Halogens in their pure form are diatomic molecules (F2, Cl2, Br2, and I2) Chlorine is a yellow-green poisonous gas Noble Gases Noble gases have 8 valence electrons (except helium, which has only 2) •they are chemically unreactive • Colorless, odorless and unreactive; they were among the last of the natural elements to be discovered Questions? White Board Refer to Periodic Trends Review Electron Configurations and Periodic Trends Write the electron configuration and draw an orbital diagram for each element Order each group of elements or ions based on given data for each property requested on card Use the orbital diagrams to explain the pattern. (does it agree with the “trend”) Objectives Use the periodic table to write electron configurations Use the periodic table to obtain information about the properties of elements