Pathology Resident Microbiology Lecture Series
advertisement

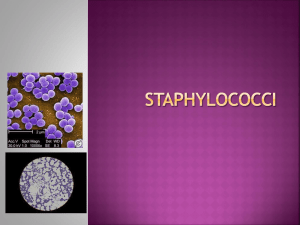
MLAB 2434: MICROBIOLOGY KERI BROPHY-MARTINEZ Staphylococci TAXONOMY Family: Micrococcaceae Genus: Staphylococcus Coagulase positive Coagulase negative Micrococcus GENUS STAPHYLOCOCCUS Coagulase positive S. aureus Coagulase negative S. epidermidis S. saprophyticus S. haemolyticus STAPHYLOCOCCUS: GRAM REACTION AND MORPHOLOGY Gram-positive spherical cells (0.5-1.5 mm) in singles, pairs, and clusters Appear as “bunches of grapes” Gram-stained smear of staphylococci from colony Scanning electron micrograph of staphylococci STAPHYLOCOCCUS: GENERAL CHARACTERISTICS Nonmotile Non–spore-forming Nonencapsulated Catalase-producing Oxidase: negative Glucose fermenters Primarily aerobic, some facultatively anaerobic STAPHYLOCOCCUS: GENERAL CHARACTERISTICS (CON’T) Bacitracin resistant Grow on agar that contains peptone Inhibited by media that has high bile salt concentration Some are ß-hemolytic Colony morphology: buttery looking, cream or white colored STAPHYLOCOCCUS AUREUS Primary pathogen of the genus Habitat: Anterior nares (carriers) Colonization: axilla, vagina, pharynx Produce superficial to systemic infections Skin Bacterial sepsis Hospital acquired infections STAPHYLOCOCCUS AUREUS Mode of transmission Traumatic introduction Direct contact with infected person Inanimate objects Predisposing conditions Chronic infections Indwelling devices Skin injuries Immune response defects STAPHYLOCOCCUS AUREUS Infection will elaborate inflammatory response with GPC accumulating as pus Pus: mix of active and inactive neutrophils, bacterial cells and extravascular fluid VIRULENCE FACTORS OF S. AUREUS Enterotoxins Cytolytic toxins Enzymes Protein A VIRULENCE FACTORS: ENTEROTOXINS Enterotoxins: Heat-stable exotoxins that cause diarrhea and vomiting Exotoxin: protein produced by a bacteria and released into environment o Heat stable @ 100 C for 30 minutes Implications Food poisoning Toxic shock syndrome Pseudomembranous enterocolitis TYPES OF ENTEROTOXINS Exfoliatin Epidermolytic toxin TSST-1: Toxic shock syndrome toxin-1 Multisystem disease Stimulates T cell production & cytokines Cytolytic Toxins Affects RBCs and WBCs Hemolytic toxins: alpha, beta, gamma, delta Panton-Valentine leukocin, lethal to WBCs VIRULENCE FACTORS: EXTRACELLULAR ENZYMES Hyaluronidase: Staphylokinase: Fibrinolysin which allows spread of infection Coagulase: Hydrolyzes hyaluronic acid in connective tissue allowing spread of infection Virulence marker Lipase: Allows colonization by acting on lipids present on the surface of the skin. VIRULENCE FACTORS: EXTRACELLULAR ENZYMES (CON’T) Penicillinase: DNase: Confers resistance Degrades DNA Beta-lactamase: Cuts the beta lactam wall of certain antibiotics VIRULENCE FACTORS: PROTEIN A Protein A: Found in cell wall Binds to Fc part of IgG Blocks phagocytosis STAPHYLOCOCCUS AUREUS: CLINICAL INFECTIONS Skin and wound Impetigo Furuncles/Boils (Infection of hair follicles usually in areas that sweat) Carbuncles (clusters of boils) Surgical wound infections Bullous impetigo STAPHYLOCOCCUS AUREUS: CLINICAL INFECTIONS (CON’T) Skin and wound Scalded skin syndrome= Ritter’s disease Extensive exfoliative dermatitis Young children and newborns Toxic Shock Syndrome Multisystem disease Caused by TSST-1 Affects women, men, and children STAPHYLOCOCCUS AUREUS: CLINICAL INFECTIONS Food poisoning Source is infected food handler Enterotoxin A the most common cause Foods affected include meat, dairy products, bakery goods with cream fillings, and salads made with eggs and mayonnaise. COAGULASE-NEGATIVE STAPHYLOCOCCI Found as indigenous flora Presence can indicate contamination Seeing an increase due to prosthetic devices, catheters and immunocompromised Abbreviated CNS or CoNS COAGULASE-NEGATIVE STAPHYLOCOCCI Habitat: Skin and mucous membranes Common human isolates S. epidermidis S. saprophyticus S. haemolyticus COAGULASE-NEGATIVE STAPHYLOCOCCI: STAPHYLOCOCCUS EPIDERMIDIS Predominantly hospital acquired infections Skin flora gets introduced by catheters, heart valves, CSF shunts Produces a slime layer that helps adherence to prosthetics and avoidance of phagocytosis UTIs are a common result COAGULASE-NEGATIVE STAPHYLOCOCCI: STAPHYLOCOCCUS SAPROPHYTICUS UTIs in young sexually active women Due in part to increased adherence to epithelial cells lining the urogenital tract Rarely present in other skin areas or mucous membranes Urine cultures If present in low amounts, it is still considered significant COAGULASE-NEGATIVE STAPHYLOCOCCI: STAPHYLOCOCCUS HAEMOLYTICUS Habitat: skin and mucous membranes Rarely implicated in infections Associated with wound infections, bacteremia, and endocarditis BREAK TIME!!! LABORATORY DIAGNOSIS: SPECIMEN COLLECTION AND HANDLING Samples must be taken from the actual site of infection Prevent delay in transport of collected material from infected sites Transport in appropriate collection device that would prevent drying and minimize growth of contaminating organisms LABORATORY DIAGNOSIS: DIRECT SMEAR EXAMINATION Microscopic Examination o Gram reaction o o Cell arrangement o o Gram-positive cocci Pairs and clusters Presence/Absence of PMNs o Numerous polymorphonuclear cells (PMNs) Insert Figure 10-1 LABORATORY DIAGNOSIS: CULTURAL CHARACTERISTICS Staphylococcus aureus Colony morphology Smooth, butyrous, white to yellow, creamy Grow well @ 18-24 hours S. aureus may produce hemolysis on blood agar S. aureus LABORATORY DIAGNOSIS: CULTURAL CHARACTERISTICS S. epidermidis Smooth, creamy, white Small-to mediumsized, usually nonhemolytic S. saprophyticus Smooth, creamy, may produce a yellow pigment IDENTIFICATION TESTS: CATALASE Principle: tests for enzyme catalase 2 H 2O 2 2 H2O + O2 Procedure Smear a colony of the organism to a slide Drop H2O2 onto smear Observe CATALASE TEST: INTERPRETATION Presence of bubbles Positive Staphylococci Absence of bubbles Negative Streptococci IDENTIFICATION TEST: SLIDE COAGULASE TEST Differentiates members within the Staphylococci Detects clumping factor found in S. aureus Procedure Place a drop of sterile water on a slide and emulsify a colony Add a drop of rabbit plasma to the suspension Observe Agglutination = Positive No agglutination= Negative IDENTIFICATION TESTS: COAGULASE TEST •Detects the extracellular enzyme “free coagulase” or staphylocoagulase •Causes a clot to form when bacterial cells are incubated with plasma •Procedure •Inoculate rabbit plasma with organism and incubate at 35-37 0 C •Observe at 30 minutes for the presence of a clot •Continue for up to 24 hours, if needed IDENTIFICATION TESTS: RAPID COAGULASE TEST Latex Agglutination Assays Detects cell-bound “clumping factor,” protein A or a combination of both Procedure Varies depending on kit type Positive reaction demonstrated by agglutination NOVOBIOCIN SUSCEPTIBILITY TEST Test to differentiate coagulasenegative staphylococci from S.saprophyticus from urine samples S. saprophyticus is resistant (top) Other CNS are susceptible MICROCOCCUS Rarely produces disease Found in environment and indigenous skin flora Catalase + Coagulase = Produces yellow pigment Microdase disc differentiate between Staph & Micrococcus Schematic Diagram for Identifying Staphylococcal Species ANTIMICROBIAL SUSCEPTIBILITY For non–beta-lactamase producing S. aureus Use pencillin Penicillinase-resistant synthetic penicillins (methicillin, nafcillin, oxacillin, dicloxacillin) Beta-lactamase producers break down the beta-lactam ring of penicillin so it inactivates antibiotic before it acts on bacterial cells METHICILLIN-RESISTANT STAPHYLOCOCCI MRSA Methicillin-resistant S. epidermidis MRSE Infection control Barrier protection Contact isolation Handwashing Treat with vancomycin Test for susceptibility with cefoxitin disk METHICILLIN-RESISTANT STAPHYLOCOCCI (CONT’D) mecA gene Encodes penicillin-binding proteins (PBPs) Causes drug ineffectiveness Gold standard Nucleic acid probe or PCR for the mec A gene VANCOMYCIN-RESISTANT STAPHYLOCOCCI VRSA= vancomycin resistant Staphylococcus aureus VISA= vancomycin intermediate Saphylococcus aureus Detection Vancomycin screening media ANTIMICROBIAL SUSCEPTIBILITY Macrolide Resistance Clindamycin sensitivity often requested by physician to treat Staph skin infection. Referred to as “D” test Clindamycin resistance is often inducible meaning it only is detectable when bacteria are also exposed to erythromycin SUMMARY MICROCOCCACEAE Staph. aureus Colony Morphology Opaque, smooth, raised, entire, whitegolden(cream) Hemolysis Most are beta hemolytic GPC in clusters, pairs, short chains or singly Pos Fermenter Non-hemolytic Staph. saprophyticus Opaque, smooth, raised, entire, butyrous, glossy, whiteyellow Non-hemolytic GPC in clusters, pairs, short chains or singly Pos Fermenter GPC in clusters, pairs, short chains or singly Pos Fermenter GPC in pairs and tetrads Neg Neg Neg Pos Resistant Resistant Resistant Sensitive Pos Neg Neg N/A Pos Neg Neg Neg Gram morphology Catalase Glucose fermentation Modified Oxidase Bacitracin susceptibility (Taxo A 0.04U) Coagulase Production (tube) Clumping factor (slide or latex Coagulase test) Staph. Epidermidis Opaque, smooth, raised, entire, graywhite Micrococcus Opaque, smooth, raised, white, bright yellow Non-hemolytic Pos Oxidizer REFERENCES Engelkirk, P., & Duben-Engelkirk, J. (2008). Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology . Baltimore, MD: Lippincott Williams and Wilkins. http://archive.microbelibrary.org/ASMOnly/Details.asp?ID=2037 http://brawlinthefamily.keenspot.com/gallery/2009-10-18-breaktime/ http://ericaandkevin.pbworks.com/w/page/5827086/Gram-Stain-and-Other-Tests http://faculty.matcmadison.edu/mljensen/111CourseDocs/111Review/Unit2Reviews/micrococcace ae_answers.htm http://jeeves.mmg.uci.edu/immunology/Assays/LatexAgglut.htm Mahon, C. R., Lehman, D. C., & Manuselis, G. (2011). Textbook of Diagnostic Microbiology (4th ed.). Maryland Heights, MO: Saunders.