Names to Formulae (3 Types)
advertisement

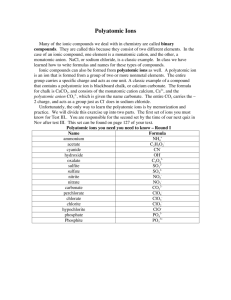
Names to Formulae (3 Types) 1. If it begins with a metal/ammonium, then a nonmetal/polyatomic ion = Ionic compound a. Write the elements or polyatomic ions in the order listed b. Find the charges of the cation and anion (check for classic/stock system use) c. Crisscross charges, reduce, using parentheses around polyatomic ion if needed 2. If it contains two nonmetals (with prefixes) = Covalent compound a. Write elements in the order listed b. Turn prefixes into that elements subscripts - do not crisscross i. if no prefix in front it is assumed to be “mono” 2. If it ends in “acid” a. Write an “H” as the cation b. Use the name to find the anion i. Hydro_____ic acid = no oxygen (either normal element or cyanide) ii. _____ic acid = anion is polyatomic ion ending in “ate” iii. _____ous acid = anion is polyatomic ion ending in “ite” c. Crisscross charges, using parentheses around polyatomic ion if needed Formulae to Names (3 Types) 1. If it begins with a metal/ammonium, then a nonmetal/polyatomic ion = Ionic compound a. For metals with only one possible oxidation state (charge) i. Write the names in the order listed ii. Change the end of compound to “ide” if not a polyatomic ion b. For metals with more than one possible oxidation state i. Write the metal indicating the present oxidation state using the classic or stock system ii. Write the anion, change the end of the compound to “ide” if not a polyatomic ion 2. If it contains two nonmetals = Covalent compound a. Write the names in the order listed b. Turn subscripts into prefixes (mono, di, tri, etc), do not write “mono” on front c. Change the end of compound to “ide” 3. If it begins with “H” - probably an acid (hydrogen peroxide is the exception) a. If it does not contain oxygen i. Form is hydro_____ic acid - anion root goes in the blank b. If it does contain oxygen i. Anion ending of “ate” = “ic”; _____ic acid ii. Anion ending of “ite” = “ous”; _____ous acid Element Bromine Carbon Chlorine Fluorine Hydrogen Iodine Nitrogen Oxygen Phosphorus Selenium Sulfur Tellurium Covalent Prefixes: Mono = 1 Di = 2 Root used in Acid Names brom -chlor fluor -iod nitr -phosphor selen sulfur -- Tri = 3 Tetra = 4 Penta = 5 Hexa = 6 Hepta = 7 Octa = 8 If name ends in “ide” bromide carbide chloride fluoride hydride iodide nitride oxide phosphide selenide sulfide telluride Nona = 9 Deca = 10 Additional Polyatomic Ions: The pattern you’ve memorized for chlorine with oxygen polyatomic ions works FOR ALL THE HALOGENS. The difference is the root above is used in place of “chlor”. ClOClO2 ClO3 ClO4 - Hypochlorite Chlorite Chlorate Perchlorate The pattern you’ve memorized for adding a single hydrogen atom to a polyatomic ion that creates a new, lower charged ion works for carbonate, sulfate and sulfite. SO42- = sulfate HSO4- = bisulfate Ionic Bonds with Classic and Stock System: The older system, called the classic system, uses the name of the metal (sometimes in Latin form) and changes the ending based on whether it is the lower charge or the higher charge. The lower charge has an ending of “ous” and a higher charge has an ending of “ic”. The newer system, called the stock system, uses roman numerals in parentheses to show the number amount of the charge. I=1 II = 2 III = 3 IV = 4 V=5 VI = 6 VII = 7 VIII = 8 IX = 9 Element Antimony Charge +3 +5 Classic System Antimonous Antimonic Stock System Antimony(III) Antimony(V) Bismuth +3 +5 Bismuthous Bismuthic Bismuth(III) Bismuth(V) Chromium +2 +3 +6 Chromous Chromic Chromium(II) Chromium(III) Chromium(VI) Cobalt +2 +3 Cobaltous Cobaltic Cobalt(II) Cobalt(III) Copper +1 +2 Cuprous Cupric Copper(I) Copper(II) Gold +1 +3 Aurous Auric Gold(I) Gold(III) Iron +2 +3 Ferrous Ferric Iron(II) Iron(III) Lead +2 +4 Plumbous Plumbic Lead(II) Lead(IV) Manganese +2 +3 +4 +7 Manganous Manganic Manganese(II) Manganese(II) Manganese(IV) Manganese(VII) Mercury +1 +2 Mercurous Mercuric Mercury(I) Mercury(II) Nickel +2 +3 Nickelous Nickelic Nickel (II) Nickel (III) Tin +2 +4 Stannous Stannic Tin (II) Tin (IV)