There are three categories for naming compounds and writing
advertisement

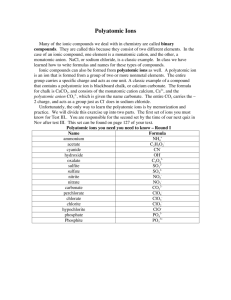
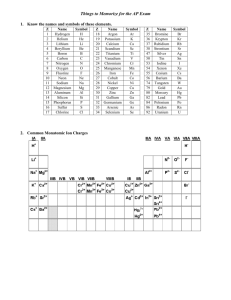
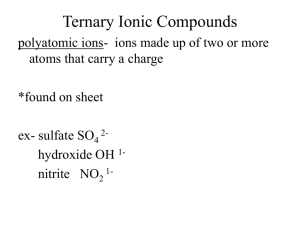
Topic E Chemical Nomenclature (Chapter 4) (See Chapter 4 of your textbook for more information) There are three categories for naming compounds and writing formulas. They can be identified by looking at the first element in the name/formula. I. Writing the Name of a Formula of an Acid a. Acids – Start with _HYDROGEN_ and are broken into three categories and they are based upon the ___ANION__ (what comes after the hydrogen.) b. If the anion is a polyatomic ion that ends in –ate i. Change the ending to ___-IC__ followed by acid ii. Example H2SO4 1. SO4 is the polyatomic ion __SULFATE___ 2. Drop the -ate and change it to _-IC___ 3. _SULFURIC_ Acid c. If the anion is a polyatomic ion that ends in –ite i. Change the ending to __-OUS__ followed by acid ii. Example H2SO3 1. SO3 is the polyatomic ion ___SULFITE___ 2. Drop the -ite and change it to __-OUS______ 3. __SULFUROUS_ Acid d. If the anion is not an –ate or an –ite i. Put hydro in front and then change the ending to “–ic” followed by acid ii. Example H2S 1. S is the anion sulfide 2. Put the Hydro in front 3. Drop the -ide and change it to –ic. 4. __SULFURIC__ Acid e. Practice: H3PO3 HI H2Se HNO3 Phosphorous Acid Hydroiodic Acid Selenic Acid Nitric Acid II. Writing the Name of a Formula of a Molecules – both elements are nonmetals a. With molecules we use prefixes to describe the number of each element present. i. Mono- Di- Tri- Tetra- Penta- Hexa- Hepta- Octa- Nona- Decaii. Except we don’t use Mono if there is only one of the first element iii. Change the second word’s ending to –ide b. Example N2Cl4 is Dinitrogen tetrachloride c. Practice: SO2 PCl5 O2F2 Sulfur dioxide Phosphorous pentachloride Dioxygen difluoride III. Writing the Name of an Ionic Compound – the first element is a METAL a. Metals take their own name i. Alkali Metals are always considered +1 and Alkaline Earth are +2 ii. Some metals have multiple charges on the periodic table and their charge must be expressed in the name as a Roman Numeral 1. Fe+2 Iron ( II ) 2. Fe+3 Iron ( III ) b. The second part is either a nonmetal or a polyatomic ion. i. Nonmetals change the ending to –ide ii. Polyatomic Ions take their own name c. Examples i. CaCl2 Calcium Chloride ii. K3PO4 Potassium Phosphate iii. FeCl2 1. Cl is always a –1 charge 2. So Iron must be a +2 charge to balance it. 3. Iron ( II ) Chloride IV. Making Formulas from Names a. Molecules are based upon the prefixes only. i. Trinitrogen Decachloride N3Cl10 ii. Silicon Dioxide SiO2 b. Ionic compounds are based upon the charges of the ions. i. The total charge of the compounds must be neutral. ii. Calcium Chloride CaCl2 iii. Aluminum Sulfite Al2SO3 iv. Chromium (III) Oxide Cr2O3 c. Acids are also based upon the charges balancing out. i. Acids ending in –ic came from polyatomic ions ending in –ate Chromic Acid H +1 CrO4-2 H2CrO4 ii. Acids ending in –ous came from polyatomic ions ending in –ite Nitrous Acid H+1 NO2-1 HNO2 iii. Acids starting with hydro come from –ide nonmetals Hydronitric Acid H+1 N-3 H3N